Preparation method of zinc-cobalt-nickel battery positive electrode composite material
A composite material, nickel battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of low capacity and poor stability of zinc-cobalt-nickel batteries, and achieve the effects of good stability, rich materials, and excellent electrochemical performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Pretreatment of nickel foam
[0038] Cut 3*5cm 2 The nickel foam was immersed in 3 mol / L dilute hydrochloric acid for 10 min to remove the nickel oxide on the surface, then transferred to 50 ml of absolute ethanol, ultrasonicated for 15 min to remove the dilute hydrochloric acid on the surface, and then dried in a blast drying oven at 60 °C spare.
[0039] (2) Preparation of cobalt phosphate
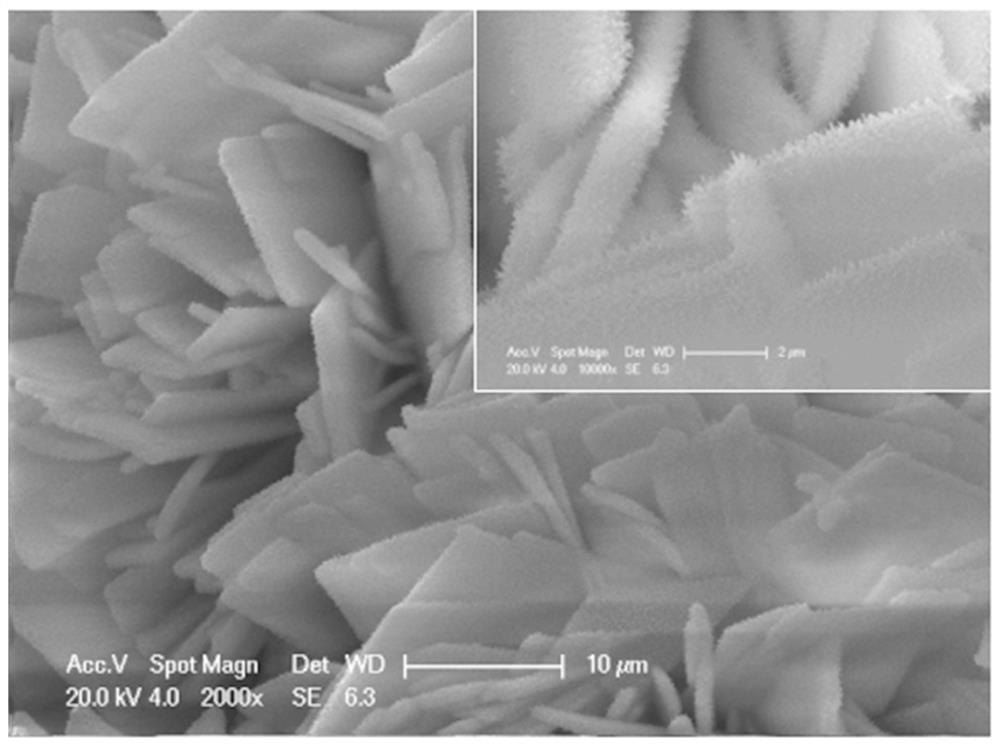
[0040] 1.8 mM cobalt nitrate, 1.2 mM ammonium dihydrogen phosphate, and 4 mM urea were fully dissolved in 80 mL of deionized water, stirred at room temperature, the resulting solution was transferred to a hydrothermal kettle, and the pretreated nickel foam ( 3 × 5 cm 2 ) was immersed in the solution in the hydrothermal kettle, sealed, and then placed in a blast drying oven for hydrothermal reaction at 120°C for 6 hours. After the reaction, the sample was taken out, and the substances on the surface were washed with deionized water and anhydrous ethanol in turn, placed in a...
Embodiment 2
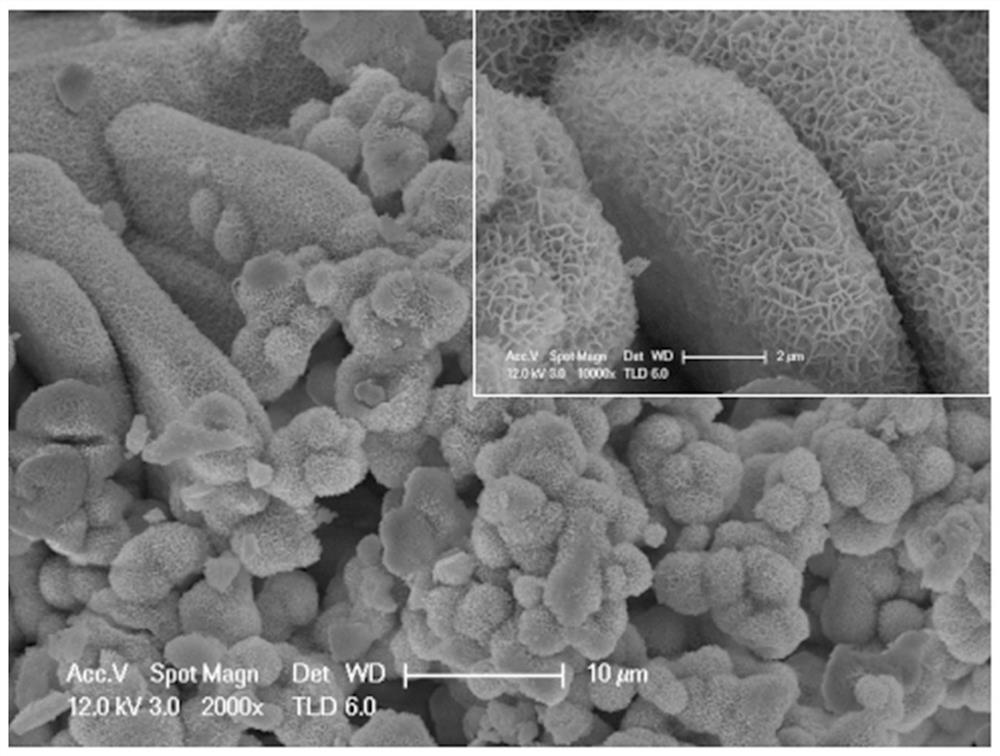
[0042] Fully dissolve 1 mM nickel nitrate, 1 mM cobalt nitrate, 6 mM ammonium fluoride and 15 mM urea in 70 ml of deionized water, stir at room temperature for 2 h to fully dissolve, transfer the resulting solution to the reaction kettle, and then put it into the The cobalt phosphate octahydrate material obtained in Example 1 was sealed and placed in a blast drying oven for hydrothermal reaction at 120°C for 5 hours. After the reaction, the sample was taken out, and the surface was washed with deionized water and absolute ethanol in turn. The existing substances were placed in a blast drying oven and kept at 60°C for 6h. image 3 For the SEM of the sample, it can be seen that the sample is grown on cobalt phosphate octahydrate with a nano-sheet layered structure.
Embodiment 3
[0044] Fully dissolve 1 mM nickel nitrate, 1 mM cobalt nitrate, 0.5 mM sodium molybdate, 6 mM ammonium fluoride and 15 mM urea in 70 ml of deionized water, stir at 35°C for 2 h to fully dissolve, and transfer the resulting solution to a Then put the cobalt phosphate octahydrate material obtained in Example 1 into the reaction kettle. After sealing, it was placed in a blast drying oven, and hydrothermally reacted at 120 ° C for 5 hours. After the reaction, the sample was taken out, and deionized Wash the substances present on the surface with water and anhydrous ethanol, place it in a blast drying oven, keep it at 60°C for 6 hours, and dry it for later use. The sample is marked as ( MCN-LDH@CP-0.5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com