Method for synthesizing unsaturated ring halide
An unsaturated and halide technology, applied in the field of synthesizing unsaturated ring halide, can solve the problem of high preparation cost, and achieve the effect of reducing preparation cost, high yield and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of methyl 4-bromo-1-methylpyrazole-3-carboxylate
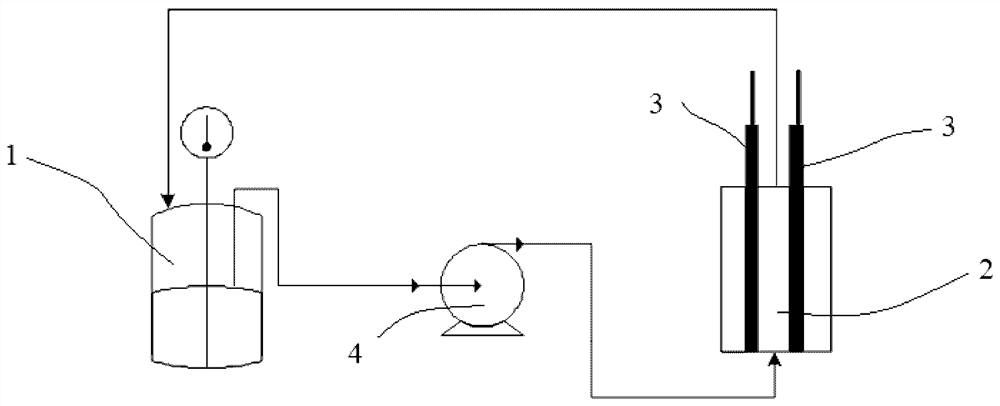
[0038] Magnons were added to a non-membrane electrolytic cell (10 mL) at room temperature of 25 °C, followed by the substrate 1-methylpyrazole-3-carboxylate methyl ester (70.1 mg, 0.5 mmol) and bromide to the cell. Sodium (77.3 mg, 0.75 mmol, 1.5 eq.). Acetonitrile (4 mL) and purified water (1 mL) were added using a syringe, followed by acetic acid (60.0 mg, 1 mmol, 2 eq.) using a microsyringe. After all the materials were added, the pH value of the reaction solution was 4, and the cathode and anode were equipped with graphite sheets (1×7×0.3cm 3 ), the electrode immersion area (that is, the area immersed in water and opposite to the other pole, the same in the following embodiments) is 1 cm 2 . The reaction solution was stirred and mixed, and 6 F was electrolyzed under a constant current of 100 mA. After the reaction, water and ethyl acetate were added for extraction and separation. The organic phase wa...
Embodiment 2
[0041] Preparation of 3-bromoindole
[0042] A non-membrane electrolytic cell (10 mL) was charged with magneton at room temperature of 25°C, followed by substrate indole (59.2 mg, 0.5 mmol), sodium bromide (77.3 mg, 0.75 mmol, 1.5 eq. ). Acetonitrile (4 mL) and purified water (1 mL) were added using a syringe. After all the materials were added, the pH value of the reaction solution was 7, and the cathode and anode were equipped with graphite sheets (1×7×0.3 cm 3 ), the electrode immersion area is 1 cm 2 . The reaction solution was stirred and mixed, and 4 F was electrolyzed under a constant current of 100 mA. After the reaction, water and ethyl acetate were added for extraction and separation. The organic phase was dried with anhydrous sodium sulfate and then concentrated. The target product was obtained by column chromatography, and the isolated yield was 90.7%. The reaction equation is as follows:
[0043] Formula (2)
Embodiment 3
[0045] Preparation of methyl 3-bromoindole-5-carboxylate
[0046] A non-membrane electrolytic cell (10 mL) was charged with magnons at room temperature of 25°C, followed by the substrate indole-5-carboxylate methyl ester (87.6 mg, 0.5 mmol), sodium bromide (77.3 mg) to the cell. , 0.75 mmol, 1.5 eq.). Add acetonitrile (4 mL) and purified water (1 mL) using a syringe. After all the materials were added, the pH value of the reaction solution was 7, and the cathode and anode were equipped with graphite sheets (1×7×0.3 cm 3 ), the electrode immersion area is 1 cm 2 . The reaction solution was stirred and mixed, and 4 F was electrolyzed under a constant current of 100 mA. After the reaction, water and ethyl acetate were added for extraction and separation. The organic phase was dried with anhydrous sodium sulfate and then concentrated. The target product was obtained by column chromatography, and the isolated yield was 87.1%. The reaction equation is as follows:
[0047] Fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com