Preparation method of non-noble metal oxygen reduction catalyst
A non-precious metal and catalyst technology, applied in the field of electrochemical catalytic energy materials, to achieve good oxygen reduction activity, avoid agglomeration, and improve the effect of low catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
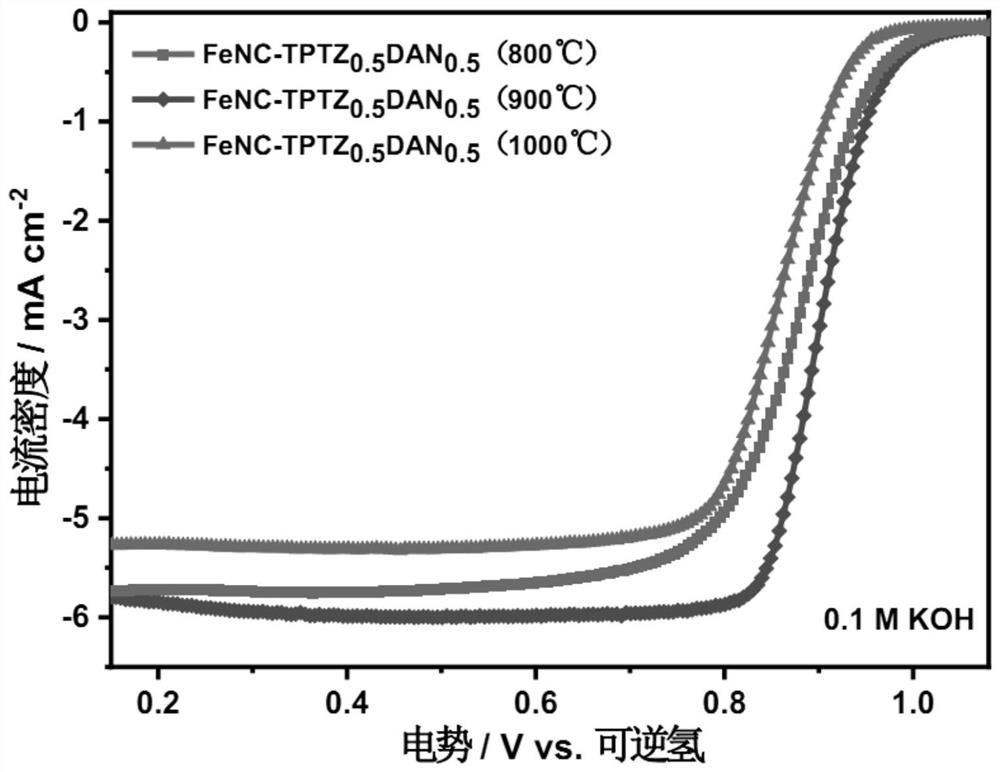
Embodiment 1
[0038] A preparation method of a non-precious metal oxygen reduction catalyst, the specific operation steps are as follows:
[0039] (1) Put 0.5 g of 2,4,6-tris(2-pyridyl)-1,3,5-triazine in a beaker, add 50 ml of absolute ethanol, stir until completely dissolved (solution A), weigh Take 0.254g of 1,8-diaminonaphthalene and add it to solution A until it is completely dissolved (solution B), then weigh 0.96g of ferrous chloride tetrahydrate and dissolve it in 20 ml of absolute ethanol to obtain a ferrous chloride solution, Namely solution C, solution C was added to solution B at one time, and the polymerization reaction was carried out with magnetic stirring at room temperature for 12 hours to form a black precipitate; The molar ratio of 1,3,5-triazine is 1:3;
[0040] (2) vacuum filtration of the obtained black precipitate after the reaction in step (1), the collected precipitate was added into a 100-milliliter ball-milling jar, 25 grams of sodium chloride metal salt was added...
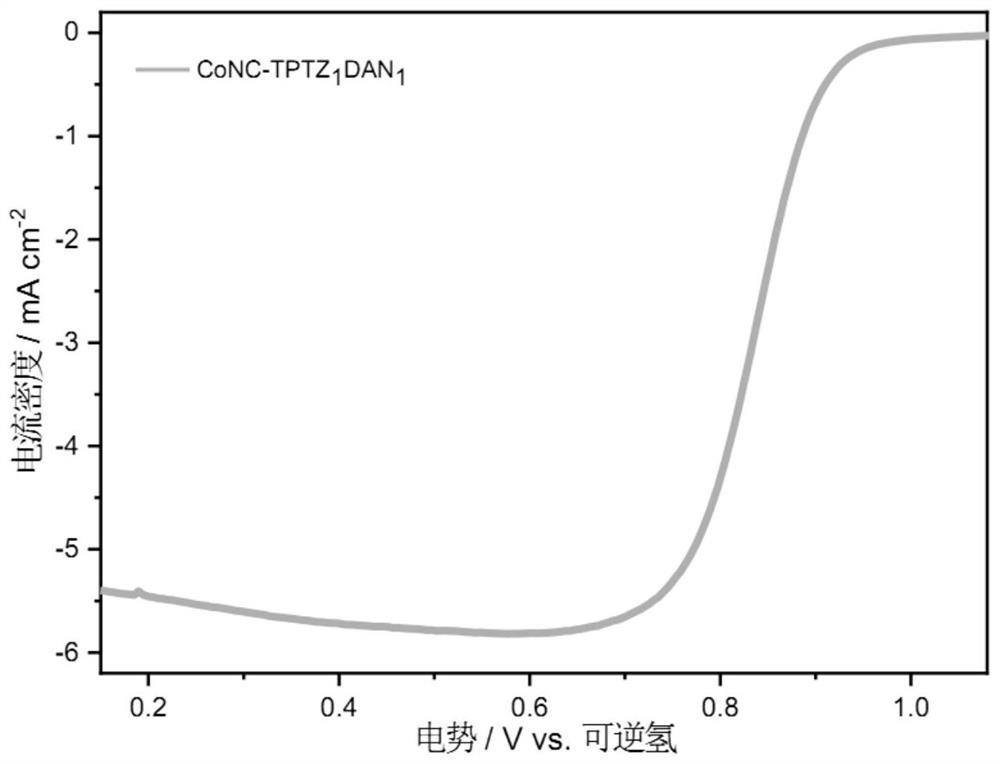
Embodiment 2
[0050] A preparation method of a non-precious metal oxygen reduction catalyst, the specific operation steps are as follows:
[0051] (1) Put 0.5 g of 2,4,6-tris(2-pyridyl)-1,3,5-triazine in a beaker, add 50 ml of absolute ethanol, stir until completely dissolved (solution A), weigh Take 0.254g of 1,8-diaminonaphthalene and add it to solution A until it is completely dissolved (solution B), then weigh 1.14g of cobalt chloride tetrahydrate and dissolve it in 20 ml of absolute ethanol to obtain a cobalt chloride solution, that is, a solution C, the solution C is added to the solution B at one time, and the polymerization reaction is carried out with magnetic stirring at room temperature for 10 hours to generate a dark green precipitate; wherein, the molar ratio of the cobalt ion in the solution C to the pyridine ring nitrogen-containing transition metal ligand is 1:3;
[0052] (2) vacuum filtration of the obtained dark green precipitate after the reaction in step (1), collect the...
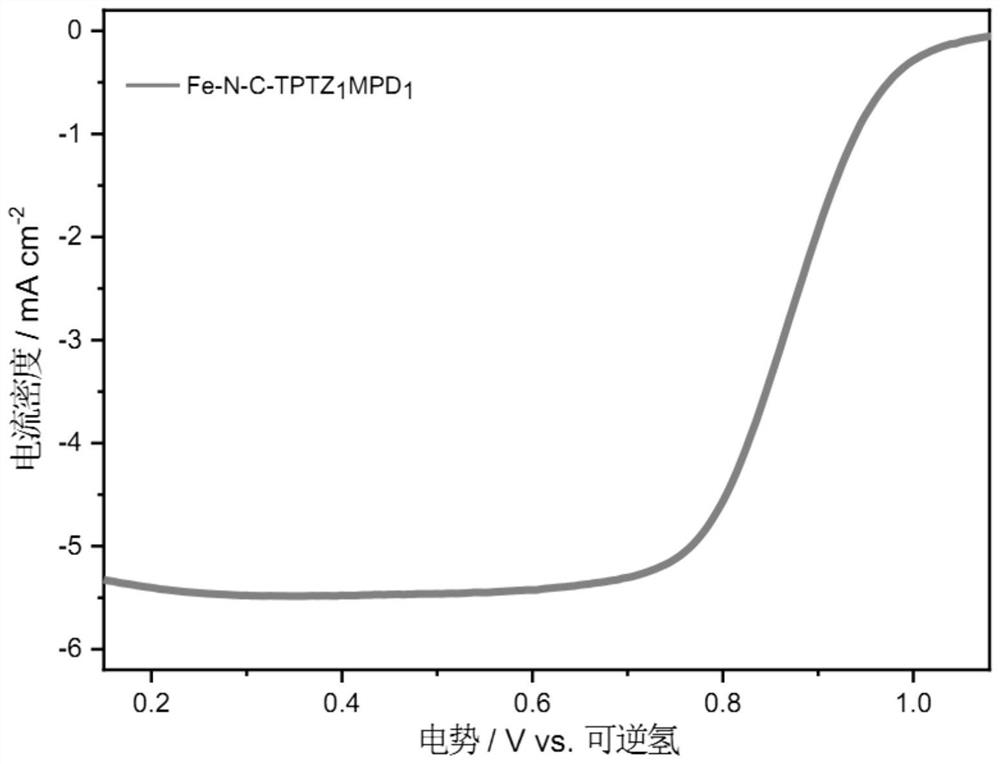
Embodiment 3
[0057] A preparation method of a non-precious metal oxygen reduction catalyst, the specific operation steps are as follows:
[0058] (1) Put 0.5 g of 2,4,6-tris(2-pyridyl)-1,3,5-triazine in a beaker, add 50 ml of absolute ethanol, stir until completely dissolved (solution A), weigh Take 0.173g m-phenylenediamine and add it to solution A, until it is completely dissolved (solution B), then weigh 1.14g of ferrous chloride tetrahydrate and dissolve it in 20 ml of absolute ethanol to obtain a ferrous chloride solution, namely solution C , the solution C was added to the solution B at one time, and the polymerization reaction was carried out with magnetic stirring at room temperature for 10 hours to form a dark green precipitate; The molar ratio of 3,5-triazine is 1:3;
[0059] (2) vacuum filtration of the obtained dark green precipitate after the reaction in step (1), collecting the precipitate and adding in 100 milliliters of ball mill jars, adding 25 grams of sodium chloride me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com