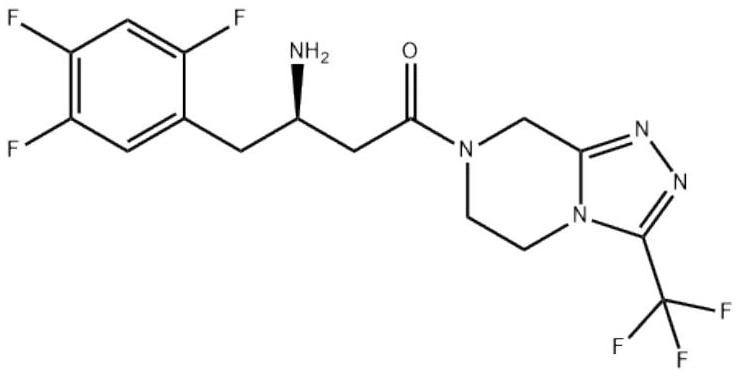
Sitagliptin phosphate pharmaceutical preparation and preparation method thereof
A sitagliptin phosphate and pharmaceutical preparation technology, which is applied in the field of sitagliptin phosphate pharmaceutical preparations and preparations, can solve the problems of complex preparation process and poor compressibility of materials, and achieve good dissolution rate and good high temperature stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
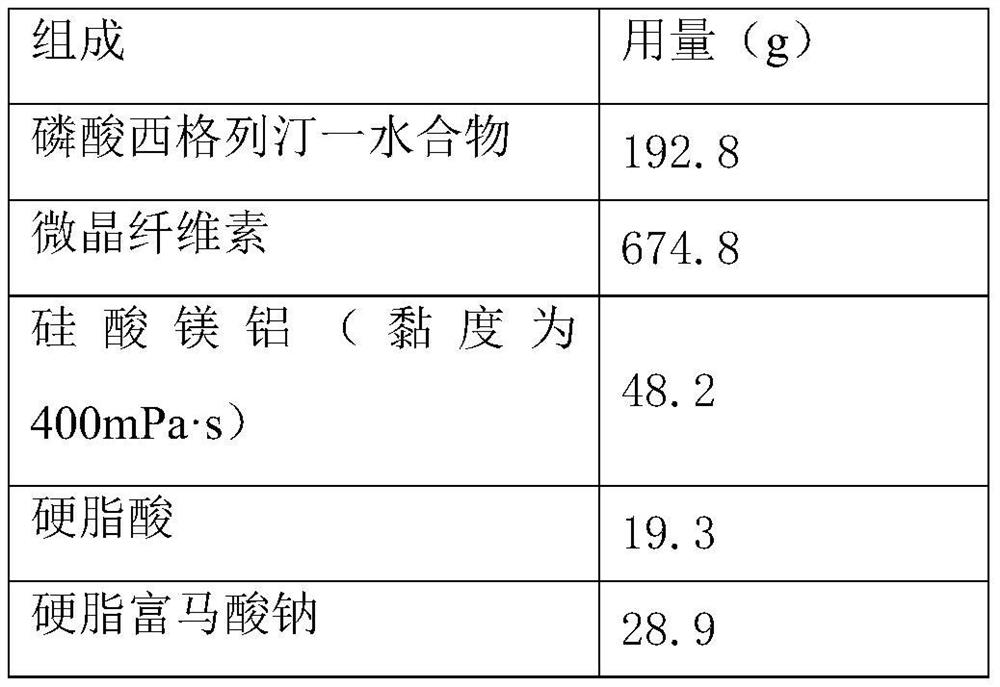
[0029] Prescription composition: the following components are the content of 1500 tablets.
[0030]
[0031] Preparation:
[0032] 1. Total mixing: Add microcrystalline cellulose, magnesium aluminum silicate (viscosity: 400 mPa·s) and sitagliptin phosphate monohydrate into the mixer and mix evenly, and then add sodium stearyl fumarate to the above mixture. The latter material is mixed uniformly, and stearic acid is added and mixed uniformly to obtain a mixture.
[0033] 2. Tablet compression: directly tablet the above mixture, the tableting process is smooth, the surface is smooth and clean, and there is no sticking phenomenon.
Embodiment 2
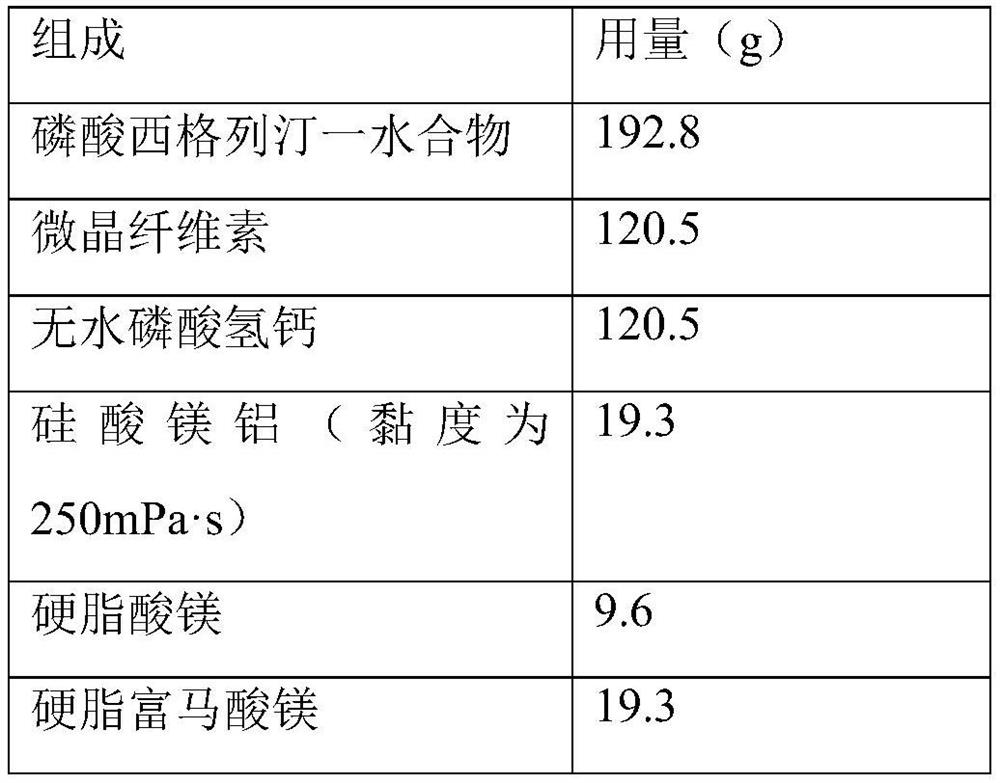
[0035] Prescription composition: the following components are the content of 1500 tablets.
[0036]
[0037] Preparation:
[0038] 1. Mixing: Add microcrystalline cellulose, anhydrous calcium hydrogen phosphate, magnesium aluminum silicate (viscosity 250mPa s) and sitagliptin phosphate monohydrate into the mixer and mix evenly, and then add stearyl fumarate. Magnesium acid is added to the above-mentioned mixed materials and mixed uniformly, and magnesium stearate is added and mixed uniformly to obtain a mixture.
[0039] 2. Tablet compression: directly tablet the above mixture, the tableting process is smooth, the surface is smooth and clean, and there is no sticking phenomenon.
Embodiment 3
[0041] Prescription composition: the following components are the content of 1500 tablets.
[0042]
[0043] Preparation:
[0044] 1. Mixing: Add microcrystalline cellulose, anhydrous calcium hydrogen phosphate, magnesium aluminum silicate (viscosity 400mPa s) and sitagliptin phosphate monohydrate into the mixer and mix evenly, and then add stearyl fumarate. Magnesium acid is added to the above-mentioned mixed materials and mixed uniformly, and magnesium stearate is added and mixed uniformly to obtain a mixture.
[0045] 2. Tablet compression: directly tablet the above mixture, the tableting process is smooth, the surface is smooth and clean, and there is no sticking phenomenon.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com