Benzylamino-substituted fatty acid derivative as well as preparation method and application thereof
A technology of fatty acid derivatives and benzyl amino, applied in the field of medicine, can solve problems such as side effects, tissue cell damage, and species reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
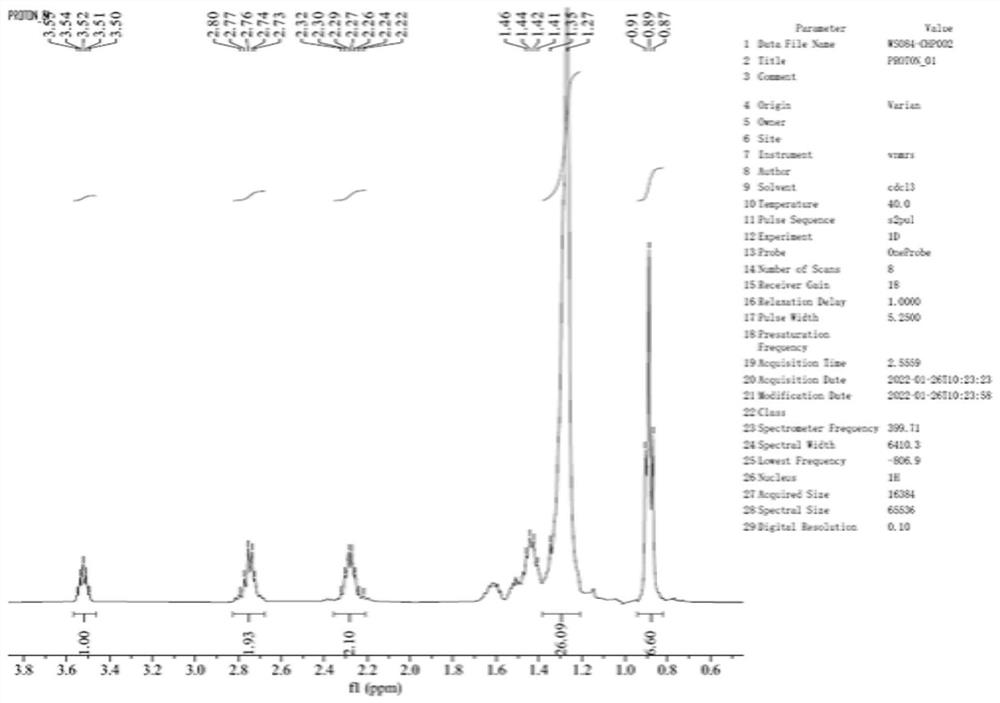
[0047] Example 1 Preparation of (R, E)-5-(benzyloxy)-2-hexylhexadec-2-enoic acid
[0048] The reaction flow is as follows:
[0049]
[0050] Specific preparation steps:
[0051] S1. Dissolve 10 g of compound I in 200 ml of chloroform, add 4.0 g of p-toluenesulfonic acid (TsOH), react at 64 ± 2 °C for 20 h, concentrate until there is no fraction, add 100 ml of dichloromethane to redissolve, wash with water until neutral, and concentrate 10g of concentrate was obtained, reconstituted with 15ml of ethyl acetate, added 75ml of n-heptane at 16°C, continued to cool to 0°C, stirred for 1 h, filtered, and the filter cake was dissolved in petroleum ether / ethyl acetate (2:1, v / v) Silica gel column chromatography was performed under the eluent system, and concentrated to obtain compound II (2.92 g).
[0052] S2. Dissolve 1.0g of compound II in 7ml of tetrahydrofuran, add 2.05ml of 2mol / l NaOH and react at 42.5±2.5°C for 87h, the reaction is complete, wash with saturated aqueous sodi...
Embodiment 2
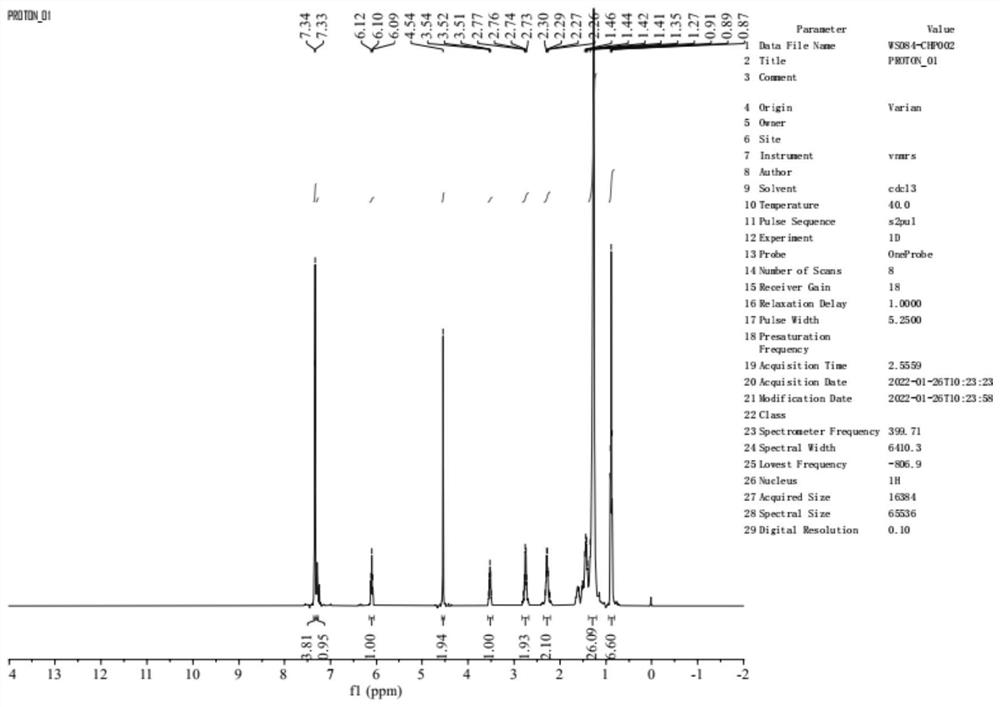
[0054] Example 2 Preparation of (R, E)-5-(benzyloxy)-2-hexylhexadec-2-enoic acid
[0055] The specific reaction process is as follows:
[0056]
[0057] S1, mix 20 g of compound I, 19.2 g of triphenylphosphine (Ph 3 P), 9.36g of p-nitrobenzoic acid were suspended in 150ml of tetrahydrofuran, 19.9g of diisopropyl azodicarboxylate (DIAD) was added at 0°C, the addition was completed, the reaction was completed for 1.0h, the reaction was completed, and 150ml of ethyl acetate was added. , washed successively with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, concentrated the organic phase to obtain 53.2 g of concentrate, and carried out petroleum ether / ethyl acetate system (2:1, v / v) column chromatography to obtain compound V ( 10.5g).
[0058] S2. Dissolve 10.5g of compound V in 60ml of methanol, add 4.35g of potassium carbonate and react at 37.5±2.5°C for 3.5h, the reaction is completed, adjust to neutrality with dilute hydroch...
Embodiment 3
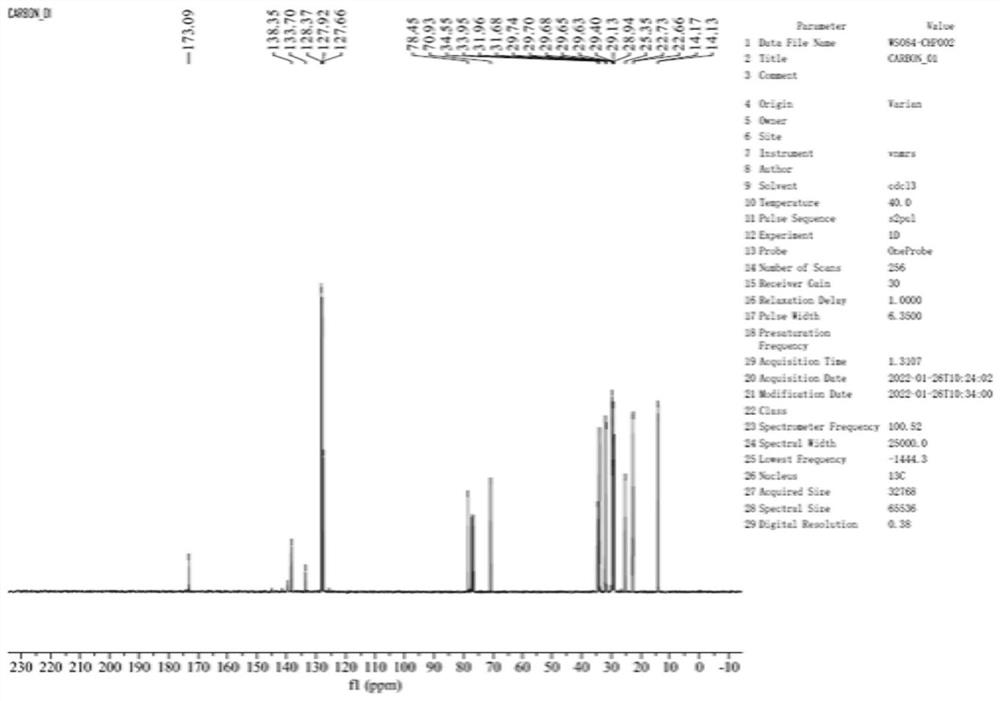
[0061] Example 3 Preparation of (R, E)-5-(benzyloxy)-2-hexylhexadec-2-enoic acid
[0062] The specific reaction process is as follows:
[0063]
[0064] S1, mix 20 g of compound I, 19.2 g of triphenylphosphine (Ph 3 P), 9.5g of N-formyl-L-leucine were suspended in 100ml of tetrahydrofuran, 19.9g of diisopropyl azodicarboxylate (DIAD) was added at 0°C, the addition was completed, the reaction was performed for 1.5h, the reaction was completed, and concentrated The reaction solution was reconstituted by adding 120 ml of n-heptane, washed with methanol aqueous solution, and then the n-heptane phase was separated and concentrated, and the concentrate was placed in petroleum ether / ethyl acetate system (2:1, v / v) silica gel column column After separation, the fractions were concentrated to obtain compound VI (12.5 g).
[0065] S2. Dissolve 12.3g of compound VI in 55ml of tetrahydrofuran, add 38ml of 1mol / l sodium hydroxide and react at 27.5±2.5°C for 3.5h, the reaction is compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com