Preparation method of 25-hydroxy-7-dehydrocholesterol
A technology for dehydrocholesterol and hydroxyl group, which is applied in the field of preparation of 25-hydroxy-7-dehydrocholesterol, can solve the problems of low product purity, complicated preparation process and high preparation cost, and achieves a simple process, environmental protection process and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
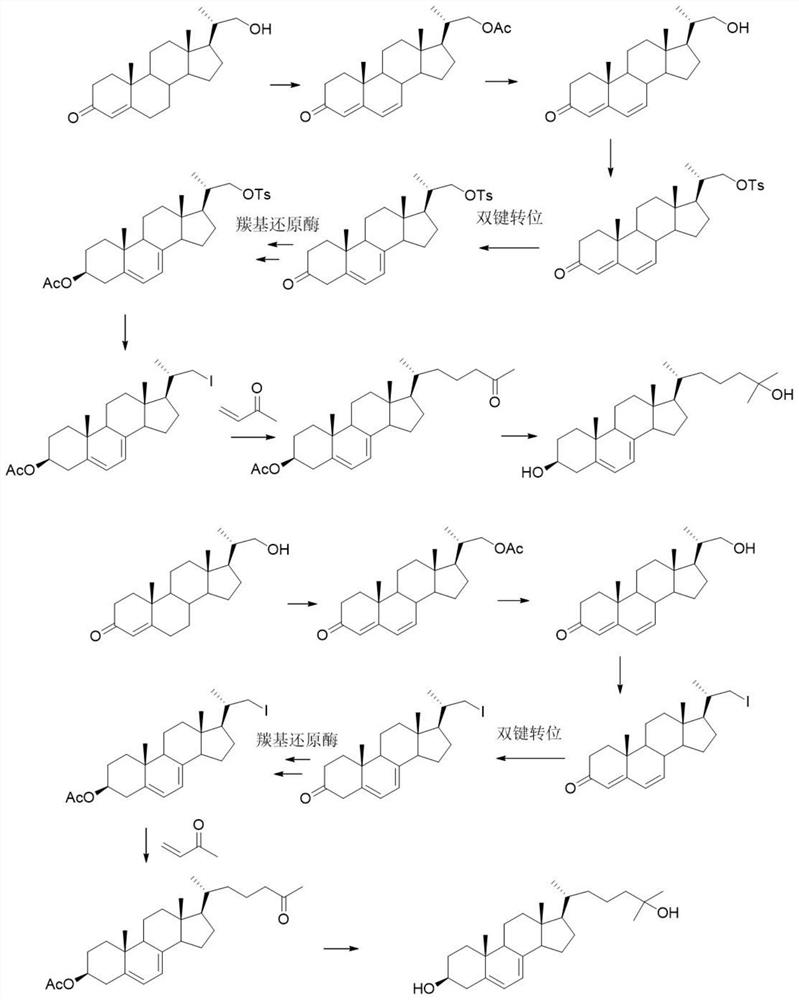
[0053] A preparation method of 25-hydroxy-7-dehydrocholesterol, the preparation method comprises the following steps:
[0054] 1) 21-hydroxy-20-methylpregna-4-en-3-one is reacted with tetrachlorobenzoquinone to obtain 21-acetoxy-20-methylpregna-4,6-diene-3 -ketone;
[0055] 2) hydrolyzing 21-acetoxy-20-methylpregna-4,6-dien-3-one to obtain 21-hydroxy-20-methylpregna-4-en-3-one;
[0056] 3) 21-hydroxy-20-methylpregna-4-en-3-one is reacted to obtain
[0057] 4) will reacted with methyl ketene to obtain
[0058] 5) will Methyl Grignard reagent and tetrahydrofuran react to give 25-hydroxy-7-dehydrocholesterol.
[0059] As a preferred version of the present invention, the reaction in the step 3) includes a sulfonation reaction, a double bond transposition reaction, a reduction reaction, an acetylation reaction and an iodination reaction that are carried out in sequence;
[0060] The sulfonation reaction is carried out by 21-hydroxy-20-methylpregna-4-en-3-one and a sulfo...
Embodiment 1
[0082] 1. Synthesis of 21-acetoxy-20-methylpregna-4,6-dien-3-one
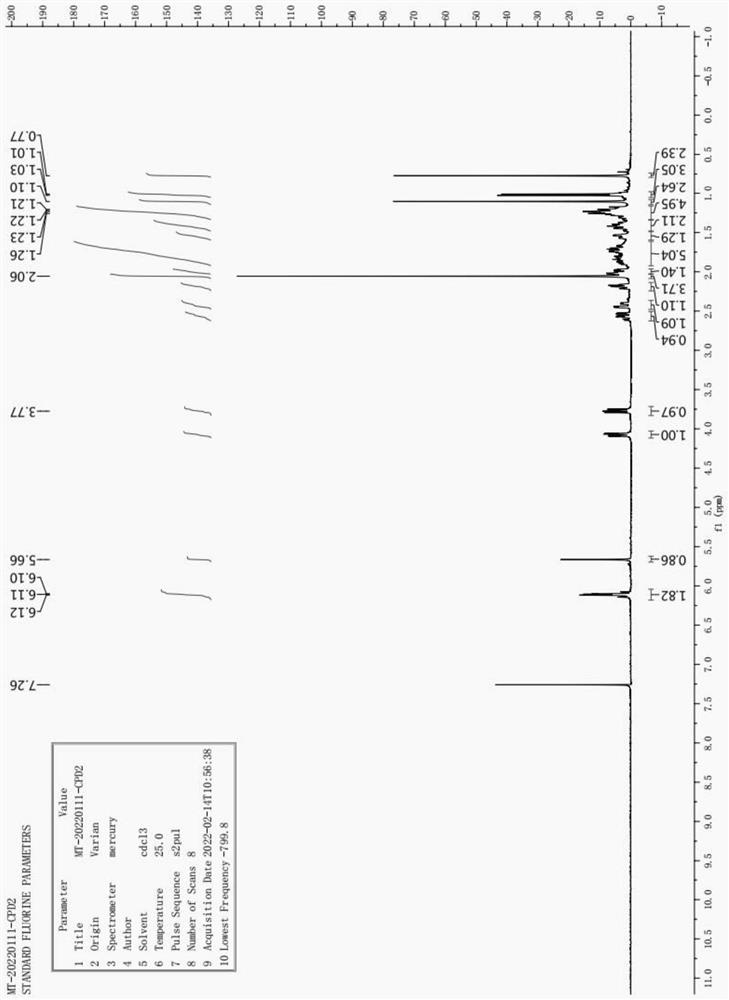
[0083] In a 2000mL three-necked flask, put 200g of 21-hydroxy-20-methylpregna-4-en-3-one, 600mL of acetic acid, 200mL of toluene, 164g of tetrachlorobenzoquinone, and react at 110°C for 6h. Cooled to room temperature, suction filtered, the filtrate was rotary evaporated to dryness, dissolved in 600 mL of acetone, added with 2000 mL of a 3.5% aqueous sodium hydroxide solution to separate out, suction filtered, and dried to obtain 199 g of product. The molar yield was 88.7%. HNMR (500MHz, CDCl 3 )δ:6.11(m, 2 H),5.66(m, 1 H),4.05(m, 1 H),3.77(m, 1 H), 2.06(s, 3 H)1.00(d, 3 H),0.98(d, 3 H),0.72(s, 3 H).
[0084] 2. Hydrolysis reaction
[0085] 199 g of 21-acetoxy-20-methylpregna-4,6-dien-3-one was dissolved in 700 mL of methanol, 200 mL of 9.7% sodium hydroxide solution was added dropwise, and the reaction was carried out at 0° C. for 2 h. After the reaction, methanol was evaporated, extracted with dich...
Embodiment 2
[0101] 1. Synthesis of 21-acetoxy-20-methylpregna-4,6-dien-3-one
[0102]In a 2000ml three-necked flask, put 200g of 21-hydroxy-20-methylpregna-4-en-3-one, 600ml of acetic acid, 200ml of toluene, 164g of tetrachlorobenzoquinone, and react at 110°C for 6h. The reaction was completed, cooled down, suction filtered, the filtrate was rotary evaporated to dryness, dissolved in 600 ml of acetone, added with 2000 ml of a 3.5% aqueous sodium hydroxide solution to separate out, suction filtered, and dried to obtain 199 g of product. The molar yield was 88.7%. HNMR (500MHz, CDCl 3 )δ:6.11(m, 2 H),5.66(m, 1 H),4.05(m, 1 H),3.77(m, 1 H), 2.06(s, 3 H)1.00(d, 3 H),0.98(d, 3 H),0.72(s, 3 H). Its spectrum is attached figure 2 shown.
[0103] 2. Hydrolysis reaction
[0104] 199 g of 21-acetoxy-20-methylpregna-4,6-dien-3-one was dissolved in 700 ml of methanol, 200 ml of sodium hydroxide solution with a mass fraction of 9.7% was added dropwise, and the reaction was stirred. After...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap