Method for continuously synthesizing peracetic acid by using microreactor
A technology of microreactor and peracetic acid, applied in chemical instruments and methods, preparation of peroxygen compounds, preparation of organic compounds, etc., can solve problems such as dangerous self-accelerating decomposition reactions, low concentration of peracetic acid, and low production efficiency , to achieve the effect of easy reaction control, short reaction time and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
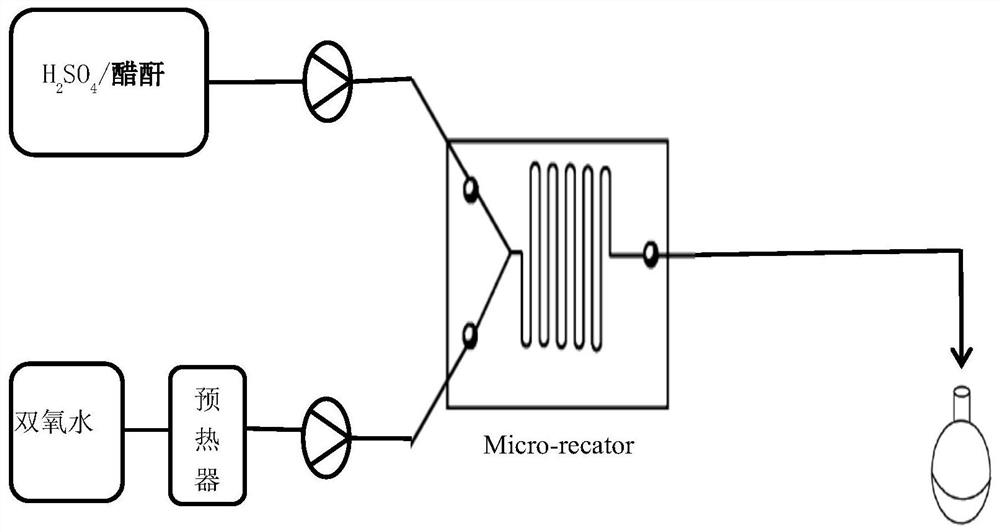
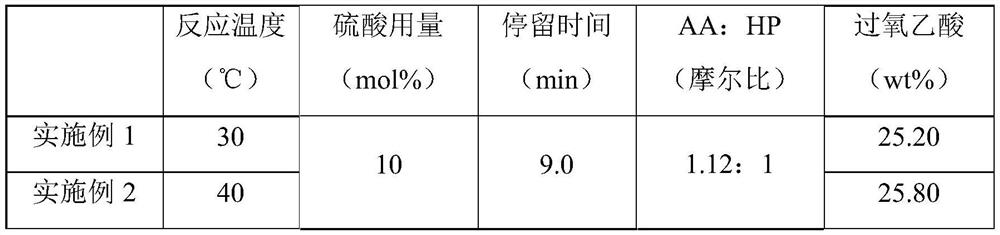
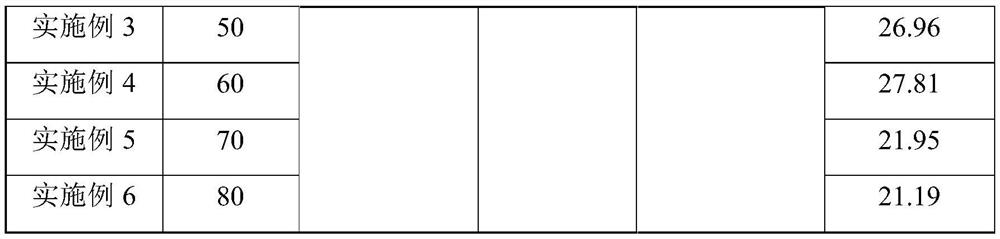
[0025] Experiments were carried out according to this method, 10mol% concentrated sulfuric acid with a concentration of 98wt% was slowly added to acetic anhydride to obtain mixed solution A, the metering pump was turned on, 30wt% hydrogen peroxide first entered the micro-preheater at a stable flow rate, and the hydraulic diameter of the micro-preheater was 1.2mm, the channel length is 35cm, the hydrogen peroxide enters the microsieve reactor after preheating, and the mixed solution A directly enters the microsieve reactor. The hydraulic diameter of the microsieve reactor is 0.5mm, and the channel length is 43cm. The mixed solution A and the hydrogen peroxide are mixed and reacted in the micro-sieve reactor. The heat exchanger controls the reaction temperature to 30-80°C, and the constant flow pump is used to control the flow rate of the reaction liquid to be 1ml / min. The residence time of the reaction is 9min. , and finally the solution obtained is analyzed according to the nat...
Embodiment 7-13
[0030] Using the same micro-preheater and micro-mesh reactor as in Example 1, acetic anhydride solution containing 10mol% concentration of 98wt% concentrated sulfuric acid and 30wt% hydrogen peroxide were input into the microreactor through two pumps for mixing and reaction, and The reaction temperature was controlled at 60°C by a heat exchanger, so that the molar ratio of acetic anhydride and hydrogen peroxide was 1.10:1, and the flow rate of the reaction solution was controlled by a constant flow pump to control the residence time of the material in the reactor to be 3.0 min and 3.6 min respectively. min, 4.5min, 6.0min, 9.0min, 12min and 18min, the product solution was collected from the discharge port, and the peracetic acid content was analyzed according to the national standard (GB 19104-2008). The results are shown in Table 2 below.
[0031]
[0032] As can be seen from the data in Table 2 above, when the residence time is 4.5min, the yield is the best, and it is give...
Embodiment 14-17
[0034] Using the same micro-preheater and micro-sieve reactor as in Example 1, acetic anhydride solution A containing 5mol%, 10mol%, and 15mol% concentration of 98wt% concentrated sulfuric acid and 30wt% hydrogen peroxide were respectively prepared into the micrometer at a stable flow rate. The reactor mixes and reacts, the heat exchanger controls the reaction temperature at 60°C, the constant flow pump is used to control the flow rate of the reaction solution, and the flow rate of the reaction solution is 1ml / min. The acetic acid content, the results are shown in Table 3 below, respectively.
[0035]
[0036] It can be seen from the above table 3 that the content of peracetic acid obtained when the amount of sulfuric acid is 15 mol% is the highest, and it is given priority to use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com