Chemical synthesis method of hirudin with tyrosine sulfation modification
A chemical synthesis, hirudin technology, applied in chemical instruments and methods, organic chemistry, peptide preparation methods, etc., to avoid side reactions and improve synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
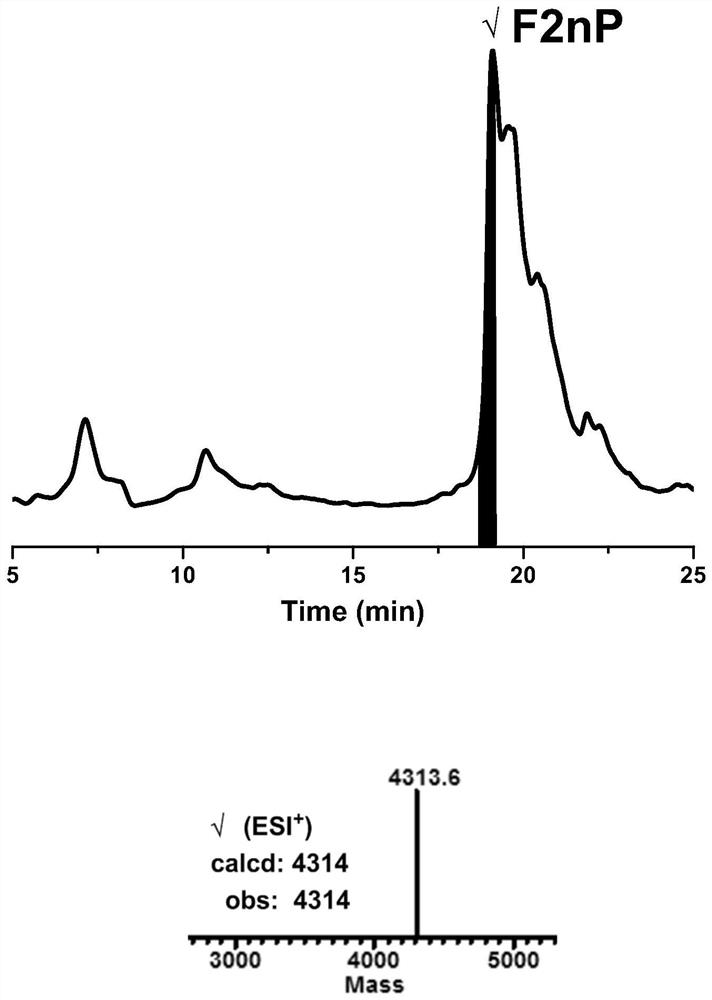
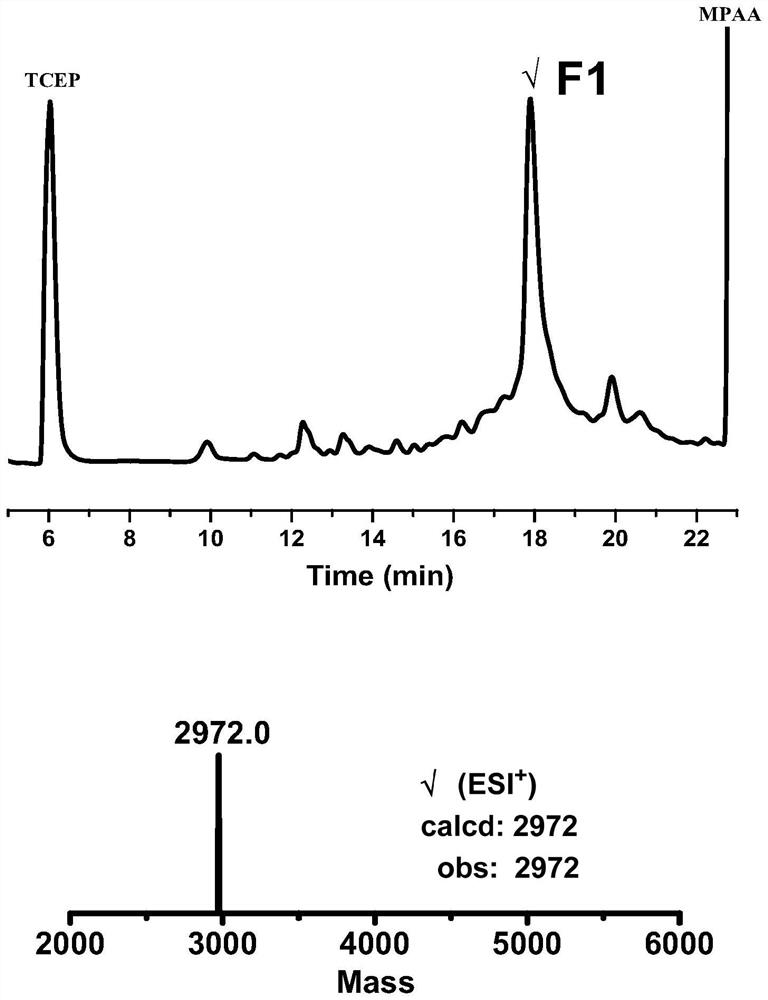
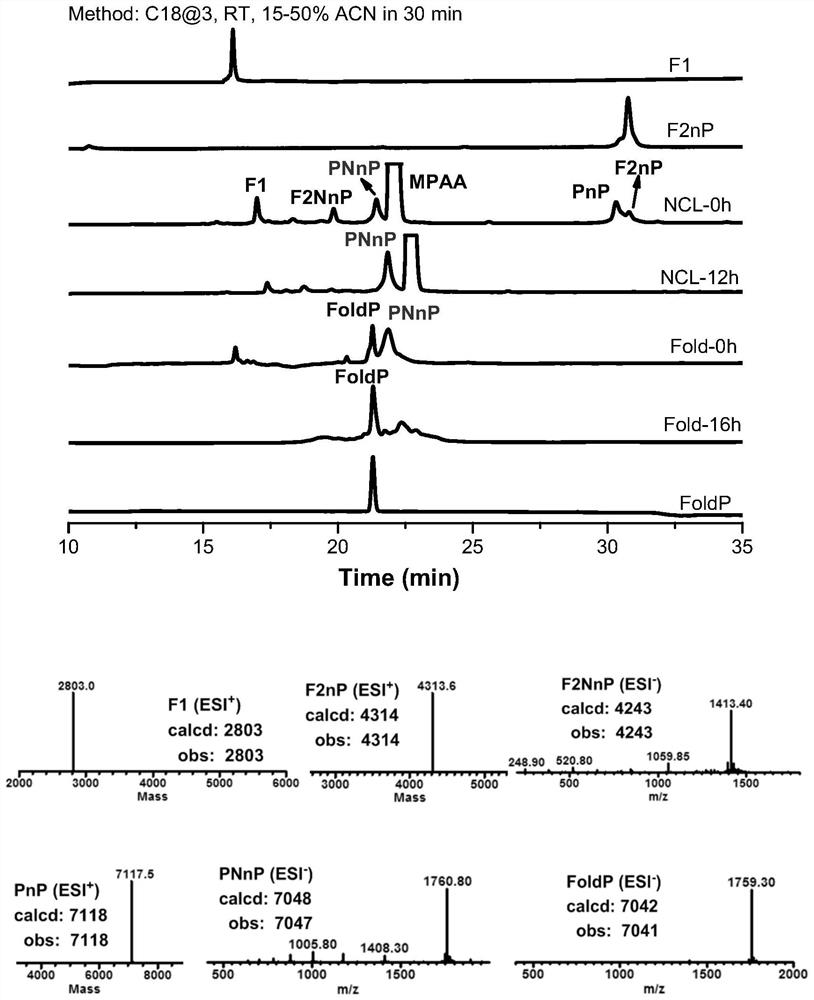
[0113] Example 1: One-pot synthesis method of ligation and refolding of hirudin HIRV1 with tyrosine sulfate modification:
[0114] (1) Fmoc-Lys(Boc)-NHNH 2 Preparation of resin: Take 1 g of 2-Chlorotryl Chloride resin (referred to as 2-Cl resin) in a peptide synthesis tube, with a loading of 0.3-0.5 mmol / g, add 15 mL of DMF to swollen twice at room temperature, 30 min each time, drain, Add 15 mL of 5% hydrazine hydrate / DMF to the resin, shake for 30 min at room temperature, wash twice with DMF, add 15 mL of 5% hydrazine hydrate / DMF for the second time, shake for 30 min at room temperature, and wash twice with DMF Then, 15 mL of 5% hydrazine hydrate / DMF was added for the third time, the reaction was shaken for 30 min at room temperature, washed twice with DMF, DCM, and DMF in turn, and the solvent was drained to obtain a resin with a hydrazide end at the C-terminus (referred to as hydrazide resin). ), add 15 mL of 5% MeOH / DMF to the resin, shake the reaction for 10 min at room...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap