Alpha-galactosidase mutant and application thereof
A technology for galactosidase and mutants, applied in the field of α-galactosidase mutants, can solve problems such as low efficiency and complex extraction process, and achieve the effects of reducing cost, high catalytic efficiency and improving transformation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
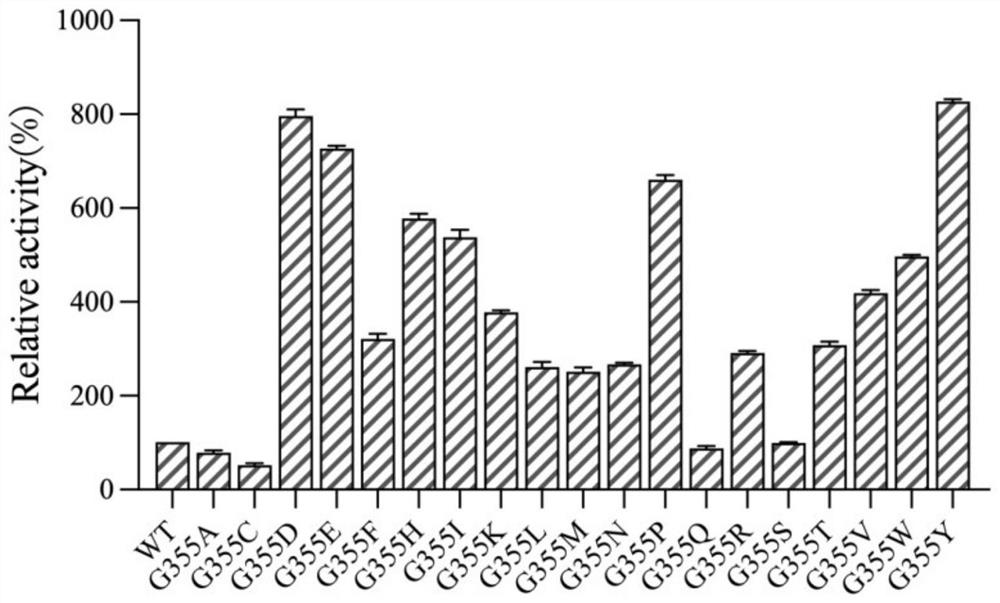
[0019] Example 1 Determination of key amino acids in the framework of α-galactosidase AgaV enzyme activity
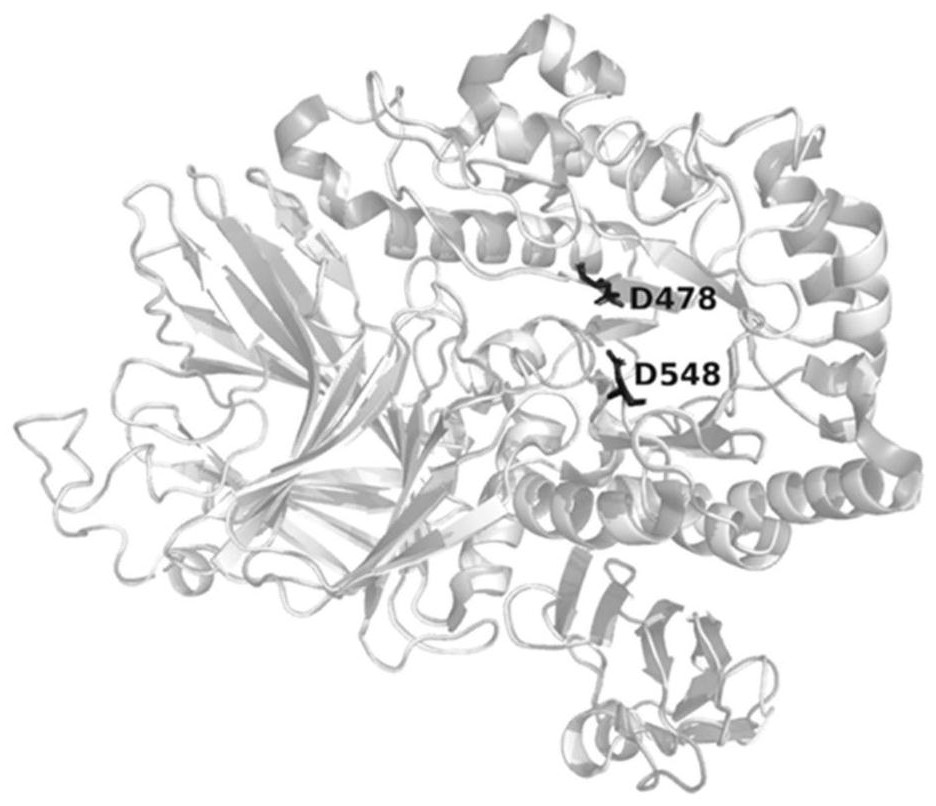
[0020] The α-galactosidase AgaV selected in the present invention has certain synthetic activity, and catalyzes the reverse hydrolysis reaction of D-galactose and glycerol to synthesize isokainate. Studies have shown that α-galactosidase, as a glycoside hydrolase, mainly catalyzes the hydrolysis reaction of glycoside compounds, and only a small part can catalyze the synthesis reaction. The present invention finds that α-galactosidase AgaB derived from Geobacillus stearothermophilus has high sequence similarity (75%) with AgaV through BLAST search, and obtains the crystal structure of AgaB in the PDB database (PDB: 4fnq), which is used as template, the three-dimensional structural model of α-galactosidase AgaV was constructed ( figure 1 ).
[0021] Through sequence alignment, it was found that the α-galactosidase AgaA and AgaB derived from Geobacillus stearothermophilu...
Embodiment 2
[0022] Example 2 Construction of α-galactosidase AgaV G355 mutant
[0023] As described in Example 1, the amino acid at position 355 of alpha-galactosidase AgaV was saturated mutated. The construction method is as follows, and the primers used are shown in the following table:
[0024] Primer Sequence(5'to 3') G355A-F CAGATCGCCGAAGCCGCAAAAGAACTGGGCATC SEQ ID NO: 3 G355C-F CAGATCGCCGAAGCCTGTAAAGAACTGGGCATC SEQ ID NO: 4 G355D-F CAGATCGCCGAAGCCGATAAAGAACTGGGCATC SEQ ID NO: 5 G355E-F CAGATCGCCGAAGCCGAAAAAGAACTGGGCATC SEQ ID NO: 6 G355F-F CAGATCGCCGAAGCCTTTAAAGAACTGGGCATC SEQ ID NO: 7 G355H-F CAGATCGCCGAAGCCCATAAAGAACTGGGCATC SEQ ID NO: 8 G355I-F CAGATCGCCGAAGCCATTAAAGAACTGGGCATC SEQ ID NO: 9 G355K-F CAGATCGCCGAAGCCAAAAAAGAACTGGGCATC SEQ ID NO: 10 G355L-F CAGATCGCCGAAGCCCTGAAAGAACTGGGCATC SEQ ID NO: 11 G355M-F CAGATCGCCGAAGCCATGAAAGAACTGGGCATC SEQ ID NO: 12 G355N-F CAGATCGC...
Embodiment 3
[0026] Example 3 Fermentation expression of recombinant α-galactosidase mutants in Escherichia coli
[0027] The specific expression method is as follows:
[0028] (1) Inoculate each G355 mutant constructed in Example 2 and the original enzyme α-galactosidase AgaV into 50 mL of LB liquid medium containing 100 μg / mL kanamycin sulfate, and culture at 37° C. and 180 rpm overnight , to prepare the seed solution.
[0029] (2) The seed liquid was inoculated into fresh 50 mL LB liquid medium with 2% inoculation amount, and cultivated to OD at 37°C and 180 rpm 600 When it is 0.6-1.0, take it out and cool it in an ice-water bath for 5 min, add the inducer IPTG (isopropyl-β-D thiogalactoside) (final concentration 0.1 mmol / L), and induce expression for 20 h at 20 °C and 180 rpm.
[0030] (3) Take the fermentation broth of induced expression, centrifuge at 12000rpm for 20min, discard the supernatant, and then use 50mM Na 2 HPO 4 -KH 2 PO 4 (pH 7.0) buffer, resuspend and wash the cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com