Citral derivative as well as preparation method and application thereof
A technology of citral and compounds, applied in the field of citral derivatives and its preparation, can solve problems such as albinism or skin cancer, irregular skin pigmentation, and browning enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
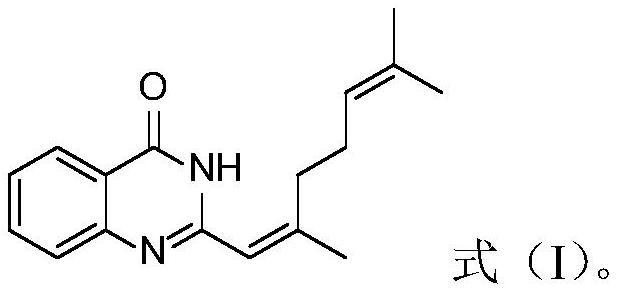
[0016] A kind of preparation method of citral derivative, its synthetic route is as follows:
[0017]
[0018] Specifically include the following steps:
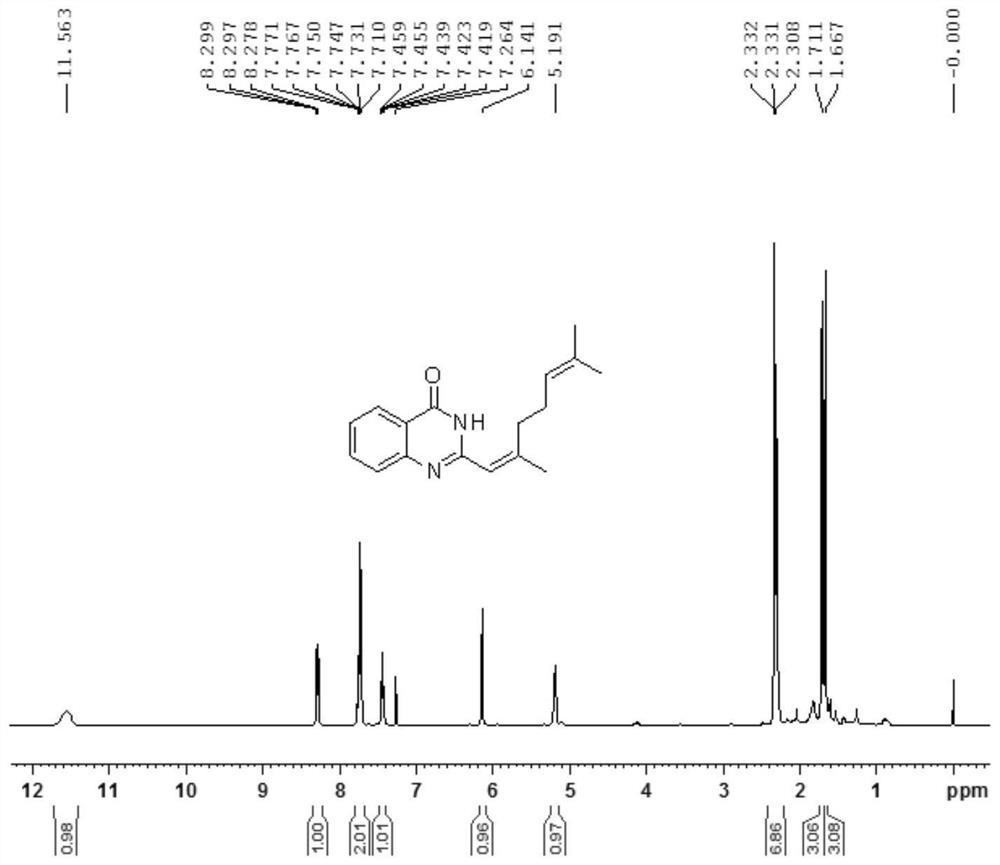
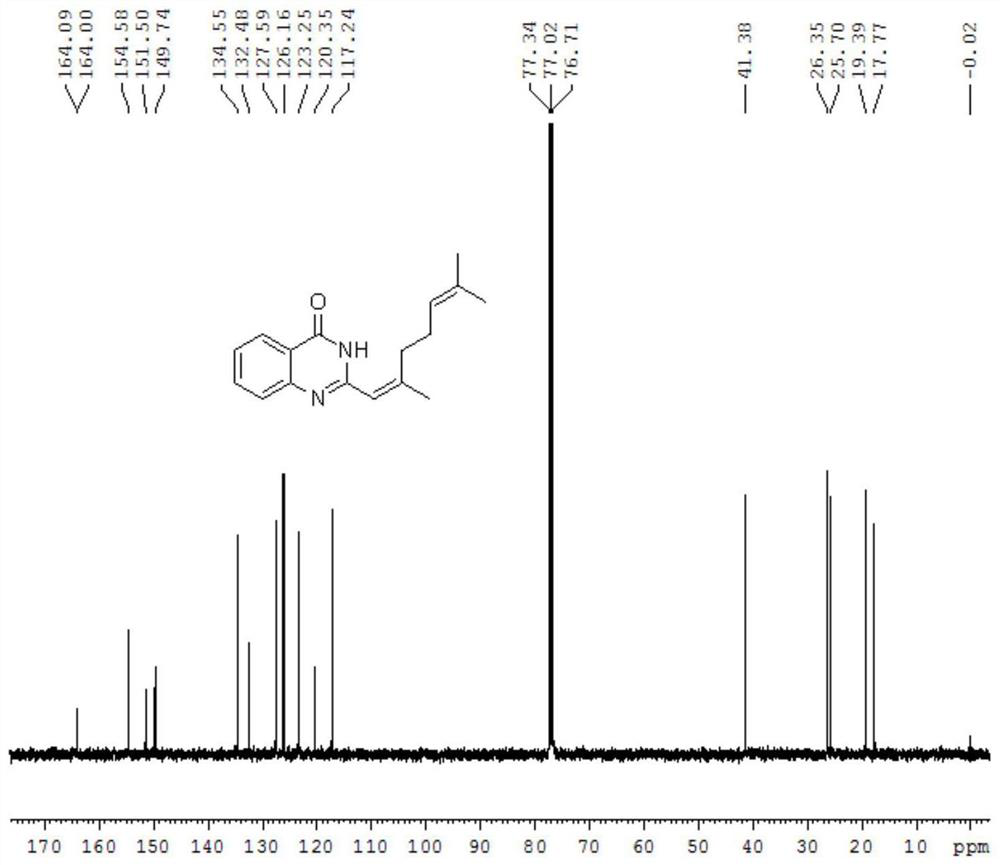
[0019] In a 50 mL round-bottom flask, 0.681 g anthranilamide (5.0 mmol), 0.913 g citral (6.0 mmol) and 10 mL DMSO were added, and the reaction was carried out at 100° C. overnight. TLC was monitored until the reaction was complete (about 20 hours). After the reaction was completed, the reaction solution was cooled to room temperature, and 10 mL of H was added. 2 O, extracted with ethyl acetate, the organic layer was dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and separated by column chromatography (eluent: petroleum ether: ethyl acetate=5:1) to obtain 0.174 g of compound 1.
[0020] test it, and the test results are as follows Figure 1-2 shown. 1 H NMR (400MHz, CDCl 3 )δ11.56(s,1H),8.33–8.24(m,1H),7.79–7.68(m,2H),7.49–7.39(m,1H),6.14(s,1H),5.19(s,1H) ,2.38–2.22(m,7H),1.71(s,3...
Embodiment 2
[0022] Diphenolase activity analysis experiment:
[0023] The specific operation is: dissolve the sample in double-distilled water to prepare sample solutions of different concentrations (citral compounds have poor water solubility, so they are dissolved in DMSO). In this experiment, the substrate was 0.5 mmol / L L-DOPA, and all manipulations were performed at 30 °C. In a 3 mL system, 0.3 mL of substrate solution, 0.05 mol / L phosphate buffer (pH 6.8) 0.75 mL, 1.8 mL of double distilled water, and 0.1 mL of sample solution with different concentrations were added to the cuvette in sequence, and finally 0.05 mL was added. The mushroom tyrosinase aqueous solution was shaken up immediately, and the curve of its absorbance value changing with time was measured using a UV-Vis spectrophotometer (wavelength was 475 nm). The slope of the obtained straight line is the enzyme activity, and the extinction coefficient is 3700L / (mol*cm) -1 Calculation. In the control group, the samples we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com