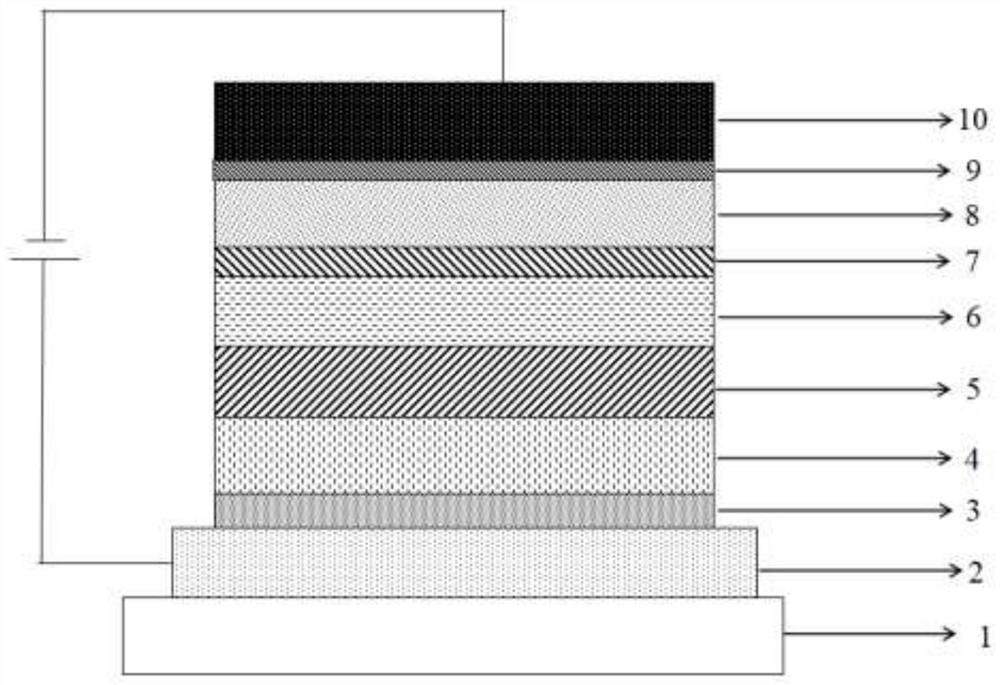
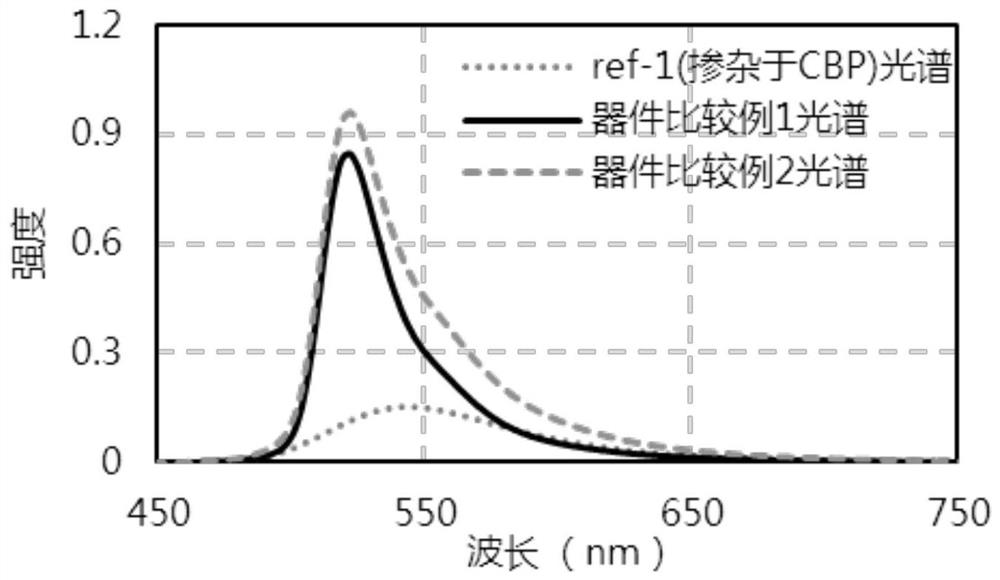
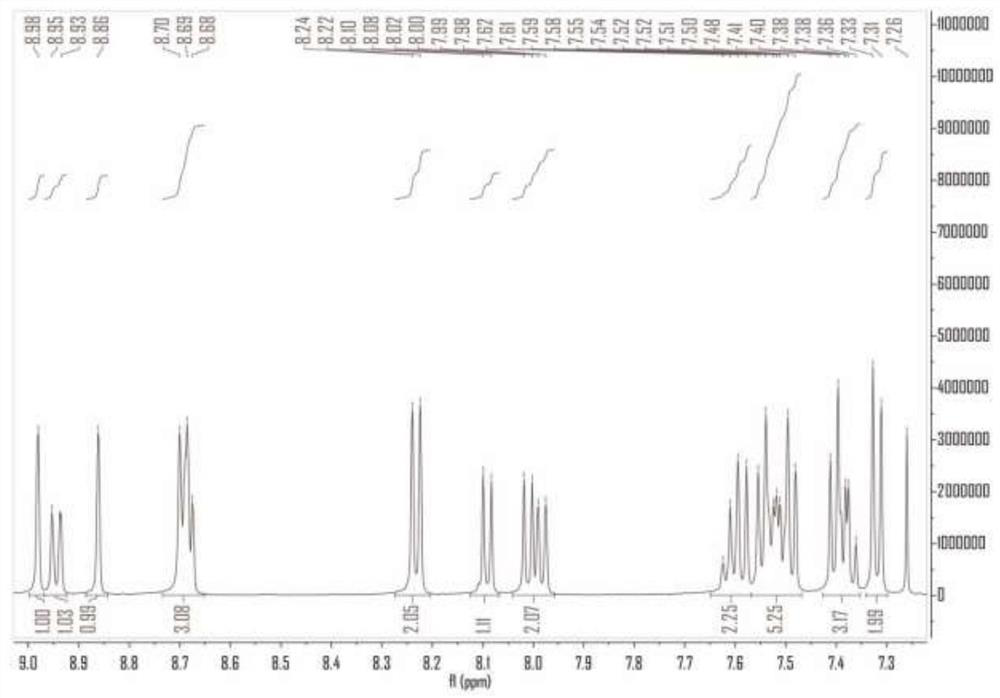
Organic compound taking triazine derivative as core and application thereof
A technology of triazine derivatives and compounds, which is applied in the field of organic compounds, can solve problems such as light leakage of the main body and the influence of device color purity, and achieve the effects of prolonging life, reducing triplet exciton quenching, and good spectral stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] Preparation of Intermediate A
[0111] The preparation process of intermediate A1:
[0112]
[0113] The starting material C1 (2.50 mmol), B 2 pin 2 (2.75mmol), PdCl 2 (dppf) 2 (0.12 mmol), KOAc (6.55 mmol) and 1,4-dioxane (10 mL) were added to a three-necked flask, and heated under reflux at 110° C. for 2 h in a nitrogen atmosphere. After cooling to room temperature, the reaction mixture was filtered through a pad of celite, rinsed with chloroform, and the resulting filtrate was evaporated in vacuo. The obtained residue was purified by silica gel column chromatography using hexane / ethyl acetate as eluent to give Intermediate A1. LC-MS: found: 248.04 ([M+H] + ), theoretical value: 247.12.
[0114] The synthesis of intermediate A2 to intermediate A3 is similar to the synthesis method of intermediate A1, the difference is that the raw materials used are different. The following table lists the structural formulas of the raw materials and products.
[0115] Tabl...
Embodiment 1
[0125] Example 1 Synthesis of Compound 1
[0126]
[0127] Add intermediate B1 (0.01mol) and intermediate A1 (0.012mol) into a three-necked flask, dissolve with mixed solvent (90ml toluene, 45ml ethanol), then add 1×10 -4 mol Pd (PPh 3 ) 4 , 3mol / L K 2 CO 3 10 mL of the aqueous solution was heated to reflux for 15 hours in a nitrogen atmosphere. Spot the plate to confirm that the reaction is complete. After cooling to room temperature, the reaction mixture was filtered through a pad of celite, rinsed with chloroform, and the resulting filtrate was evaporated in vacuo. The obtained residue was purified by column chromatography on silica gel using hexane / toluene as eluent to yield intermediate M1. LC-MS: found: 532.26 ([M+H] + ), theoretical value: 531.15.
[0128]
[0129] The intermediate M1 (0.90 mol), starting material N1 (1.35 mol), K 2 CO 3 (3.37 mmol) and DMF (10 ml) were added to a three-necked flask, and then heated to 110°C. After stirring for 2 hours,...
Embodiment 3
[0130] Example 3 Synthesis of Compound 35
[0131]
[0132] Add intermediate B2 (0.01mol) and intermediate A2 (0.012mol) into a three-necked flask, dissolve with mixed solvent (90ml toluene, 45ml ethanol), then add 1×10 -4 mol Pd (PPh 3 ) 4 , 3mol / L K 2 CO 3 10 mL of the aqueous solution was heated to reflux for 15 hours in a nitrogen atmosphere. Spot the plate to confirm that the reaction is complete. After cooling to room temperature, the reaction mixture was filtered through a pad of celite, rinsed with chloroform, and the resulting filtrate was evaporated in vacuo. The obtained residue was purified by column chromatography on silica gel using hexane / toluene as eluent to yield intermediate M2. LC-MS: found: 461.17 ([M+H] + ), theoretical value: 460.11.
[0133]
[0134] The intermediate M2 (0.90 mmol), starting material N1 (2.7 mmol), K 2 CO 3 (3.37 mmol) and DMF (10 ml) were added to a three-necked flask, and then heated to 110°C. After stirring for 2 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com