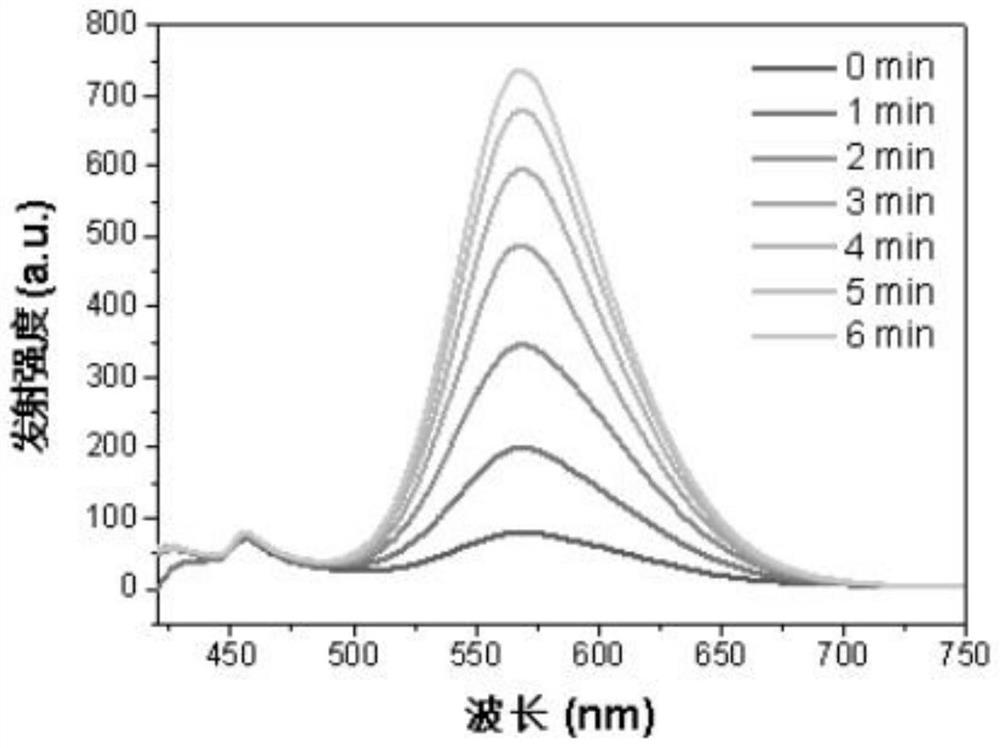
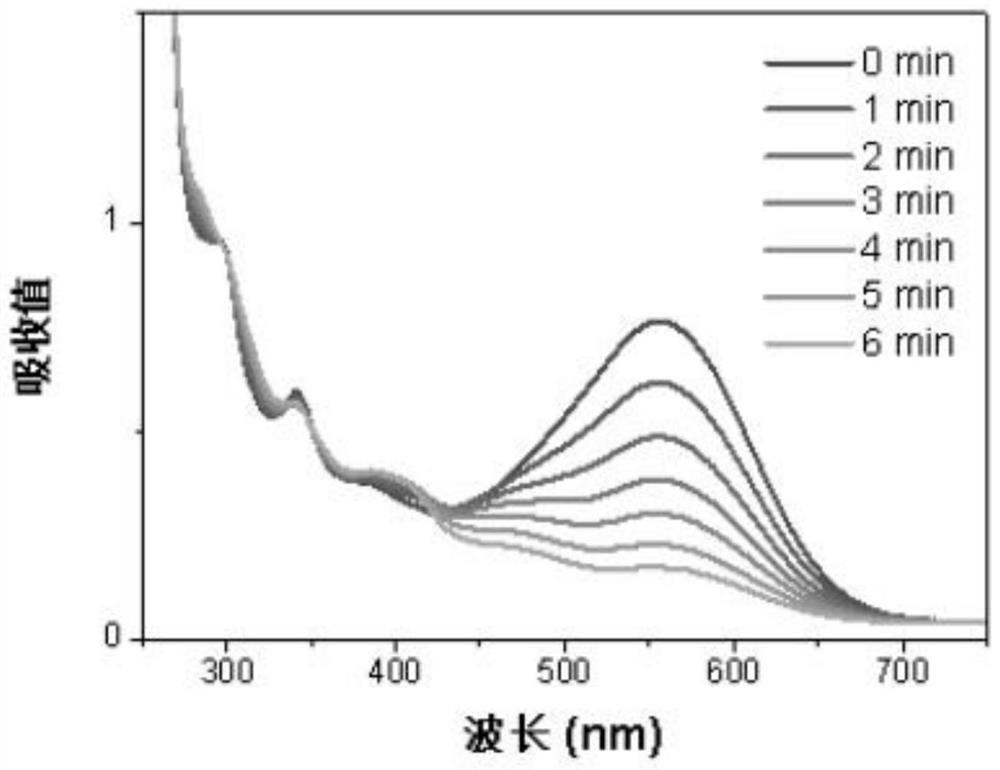
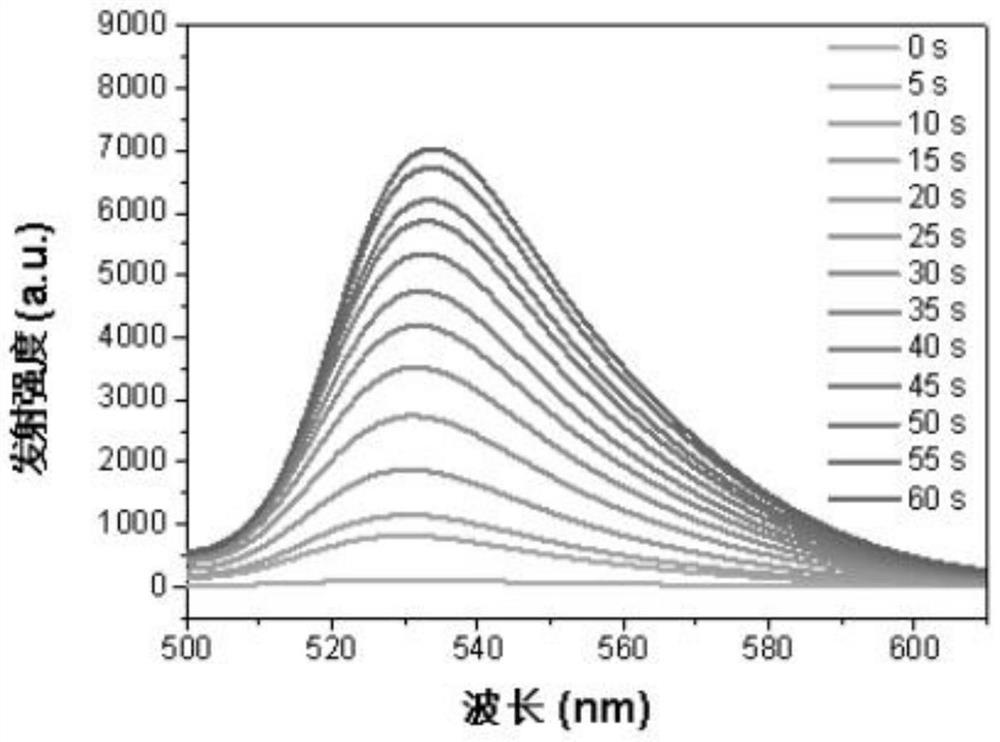
Iridium complex photosensitizer as well as preparation method and application thereof
A technology of iridium complexes and photosensitizers, applied in the field of iridium complex photosensitizers and their preparation, can solve the problems of affecting the treatment effect of PDT, ROS short life, limited diffusion distance, etc., achieve high yield, improve effect, and preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The invention provides the preparation method of the above-mentioned iridium complex photosensitizer, comprising the following steps:
[0049]S1, 1,10-phenanthroline-5,6-dione, terephthalaldehyde, ammonium acetate and acetic acid are reacted, and the pH of the mixture obtained by the reaction solution and water is adjusted to neutrality to obtain a mixture, and the mixture is sequentially Separate, dissolve, filter, and dry to obtain the ligand fmp;
[0050] S2. Under protective gas, react 2,3,3-trimethylindole, 1,3-propane sultone and o-dichlorobenzene to obtain compound 1;
[0051] S3, under protective gas, react the ligand fmp, compound 1, catalyst and mixed solvent 1, and mix the reaction solution with saturated sodium chloride solution and then separate and dry in sequence to obtain ligand psi;
[0052] S4, the IrCl 3 ·3H 2 O, 2-phenylpyridine and mixed solvent 2 react to obtain compound 2;
[0053] S5, under protective gas, react compound 2, ligand psi and mix...
Embodiment 1
[0082] 1,10-Phenanthroline-5,6-dione (2 mmol), terephthalaldehyde (4 mmol) and ammonium acetate (60 mmol) were dissolved in acetic acid (60 mL) and refluxed at 115 °C for 30 min. After cooling to room temperature, 250 mL of distilled water was added, and the pH was adjusted to neutrality with concentrated ammonia water (25% by mass) under stirring conditions, and a yellow solid was precipitated. Then carry out suction filtration, the crude product after suction filtration is washed three times with water, and the orange crude product obtained after drying at 80°C for 24 hours is then heated and dissolved in ethanol for 30min at 85°C, wherein the mass-volume ratio of the orange crude product to ethanol is 100mg: 200mL, after hot filtration, put the filtrate into a rotary dryer to spin dry to obtain the yellow ligand fmp with a yield of 70.0%;
[0083] Dissolve 2,3,3-trimethylindole (3.14 mmol) and 1,3-propane sultone (3.48 mmol) in 3 mL of o-dichlorobenzene, reflux at 120 °C fo...
Embodiment 2
[0088] IrCl 3 ·3H 2 O (2 mmol) and 2-phenylpyridine (4.4 mmol) were dissolved in 50 mL of mixed solvent (the volume ratio of ethylene glycol ether and water was 3:1), refluxed at 130 ° C for 24 h, cooled to room temperature, and then pumped After filtration, the crude product after suction filtration was dried at 80 °C for 24 h to obtain yellow compound 2 (Ir 2 (ppy) 4 Cl 2 ), the yield is 80%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com