Classical swine fever virus E2-E0 fusion protein as well as preparation method and application thereof
An E2-E0, swine fever virus technology, applied in the field of preparation of swine fever virus E2-E0 fusion protein, can solve the problems of lack of glycosylation modification, lack of expression of target protein, affecting the immunogenicity of antigenic protein, etc. To achieve the effect of increasing protein expression and promoting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
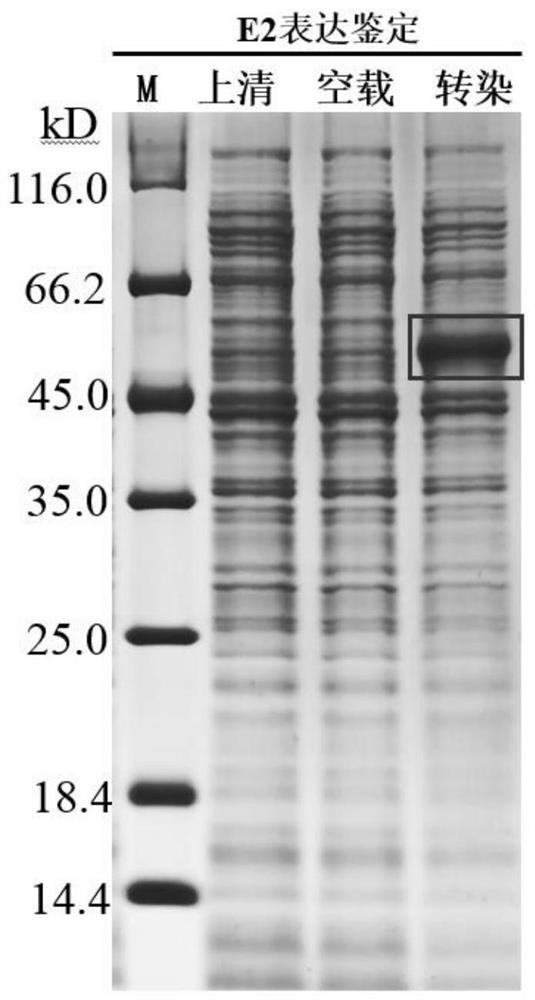
[0043] Example 1 Preparation of swine fever virus E2-E0 truncated fusion protein and E2-E0 truncated mutant fusion protein
[0044] 1. Gene Fragment Synthesis
[0045] Gene fragments encoding swine fever virus E0 protein, E0 truncated protein, E0 truncated mutant protein, E2 protein, E2-E0 truncated fusion protein and E2-E0 truncated mutant fusion protein were synthesized by Beijing Liuhe Huada Gene Technology Co., Ltd. , and was correctly identified by nucleic acid gel electrophoresis. The gene fragment encoding swine fever virus E0 protein is shown in SEQ ID NO.12, and the amino acid sequence is shown in SEQ ID NO.11; the gene fragment encoding swine fever virus E0 truncated protein is shown in SEQ ID NO.2, The amino acid sequence is shown in SEQ ID NO.1; the gene fragment encoding the swine fever virus E0 truncation mutation is shown in SEQ ID NO.8, and the amino acid sequence is shown in SEQ ID NO.7. The E0 truncated mutant protein refers to the The amino acids at positi...
Embodiment 2
[0056] Example 2 Immunogenicity detection
[0057]Indirect ELISA was used to detect mE0 protein, E2 protein, E2-E0 truncated fusion protein and E2-E0 truncated mutant fusion protein immunized rabbit serum to analyze their immunogenicity. The purified CSFV-E0 truncation mutant protein, CSFV-E2 protein, CSFV-E2-E0 truncation mutant fusion protein and CSFV-E2-E0 truncation mutant fusion protein (50ng / well) were packaged with 50 mM carbonate buffer, respectively. Incubate overnight at 4°C in an ELISA plate. Block with 1% bovine serum albumin (BSA) at 37°C for 2h. The serum to be tested was diluted with serum diluent at 1:2000 and added to the reaction well, 100 μL / well, each sample was set to 3 duplicate wells, and incubated at 37°C for 1 h. The HRP-labeled goat anti-rabbit IgG antibody was diluted with serum diluent at 1:5000 and added to the reaction well, 100 μL / well, and incubated at 37°C for 1 h. TMB color development, 50 μL per well, after 10 min of color development, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com