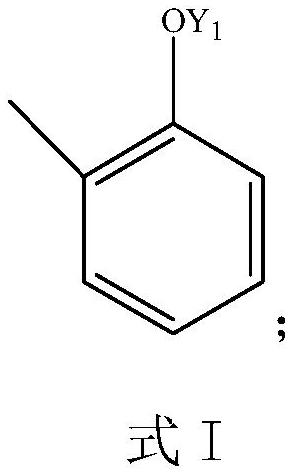
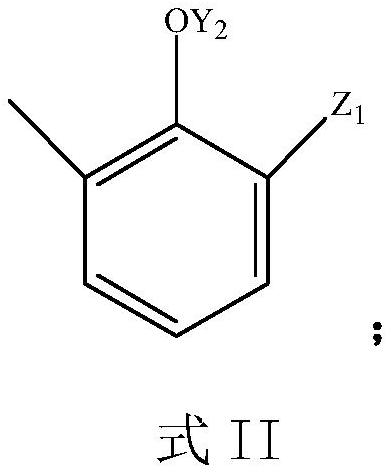
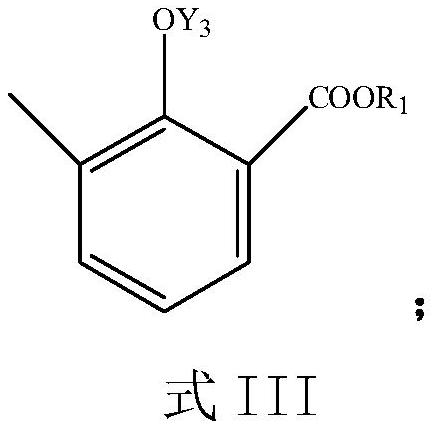
Preparation process of sodium 3-methyl salicylate
A sodium methyl salicylate and preparation technology, which is applied in carboxylate preparation, organic chemistry, bulk chemical production, etc., can solve the problem that the number of patents is small, hindering the large-scale application of 3-methyl salicylate, etc. problem, to achieve the effect of mild and not harsh reaction conditions, large-scale large-scale production and application, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A preparation process of sodium 3-methylsalicylate, comprising the following preparation steps:
[0056] (1) Preparation step 1:
[0057]
[0058] In the reactor, 1 mole of compound 1 was reacted with formaldehyde and hydrogen chloride; the substance ratio of compound 1, formaldehyde and hydrogen chloride was 1:1.1:1.1, the reaction solvent was DMF, the reaction temperature was 80°C, and the reaction pressure was 0.5 MPa, the reaction time is 4 hours, the reaction is completed, sodium hydroxide solution is added, and the reaction is continued for 4 hours. After the reaction is completed, it is lowered to room temperature, the organic phase is collected, and spin-dried to obtain compound 2.
[0059] (2) preparation step 2:
[0060]
[0061] In the reactor, 1 mole of compound 2 in the above formula was reacted with hydrogen peroxide; the substance ratio of compound 2 to hydrogen peroxide was 1:1.5; the solvent was THF, the reaction temperature was 50°C, and the rea...
Embodiment 2
[0069] A preparation process of sodium 3-methylsalicylate, comprising the following preparation steps:
[0070] (1) Preparation step 1:
[0071]
[0072] In a reactor, combine 1 mole of compound 1 with formaldehyde and Br 2 Reaction; compound 1, formaldehyde, Br 2 The amount ratio of the substances is 1:1.1:1.5, the reaction solvent is THF, the reaction temperature is 60 ° C, the reaction pressure is normal pressure, the reaction time is 6 hours, the reaction is completed, the sodium hydroxide solution is added, and the reaction is continued for 2 hours. After completion, it was lowered to room temperature, the organic phase was collected, and spun dry to obtain compound 2.
[0073] (2) preparation step 2:
[0074]
[0075] In the reactor, 1 mole of compound 2 in the above formula was reacted with potassium permanganate; the substance ratio of compound 2 to potassium permanganate was 1:1.2; the solvent was acetonitrile, the reaction temperature was 30°C, and the react...
Embodiment 3
[0083] A preparation process of sodium 3-methylsalicylate, comprising the following preparation steps:
[0084] (1) Preparation step 1:
[0085]
[0086] In the reactor, 1 mole of compound 1 was reacted with formaldehyde and thionyl chloride; the substance ratio of compound 1, formaldehyde and thionyl chloride was 1:1.5:2, the solvent was DMSO, and the reaction temperature was 60°C , the reaction pressure is normal pressure, and the reaction time is 6 hours. After the reaction is completed, sodium hydroxide solution is added, and the reaction is continued for 2 hours.
[0087] (2) preparation step 2:
[0088]
[0089] In the reactor, 1 mole of compound 2 in the above formula was reacted with ammonium persulfate; the substance ratio of compound 2 to sulfuric acid was 1:1.2; the solvent was acetone, the reaction temperature was 60°C, and the reaction pressure was normal pressure, The reaction time was 3 hours, the reaction was completed, the reaction was continued to com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com