Preparation method of oxime ether
A technology of oxime ether and p-ph, applied in organic chemistry, chemical recycling, etc., can solve problems such as metal residues, environmental and human hazards, and achieve the effects of low cost, avoiding safety hazards, good ecological advantages and economic advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Anhydrous n-butyl acetate, benzophenone oxime (0.0197g, 0.1mmol, 1.0eq), anhydrous tetrahydrofuran (0.3606g, 50eq), carbon tetrabromide (0.0597g) were sequentially added to a 5mL transparent glass reaction flask , 1.8eq), 0.1000g molecular sieve, mix well. After the magnetron was placed, the reaction flask was placed under argon for ventilation for 15 minutes, and the reaction was performed under the irradiation of a 3W LED blue light for 5 hours. After the reaction is completed, the reacted solution is placed in a centrifuge tube for centrifugation, and the upper clear liquid is taken and concentrated under reduced pressure with a rotary evaporator to obtain a thick liquid. Finally, ethyl acetate and petroleum ether were mixed in proportion as a developing solvent, and column chromatography was performed to separate the obtained product, and the yield was 87% after precipitation and drying. like figure 1 shown: 1 H NMR (400MHz, CDCl 3 )δ7.56-7.46(m,2H),7.44-7.37(m,...
Embodiment 2
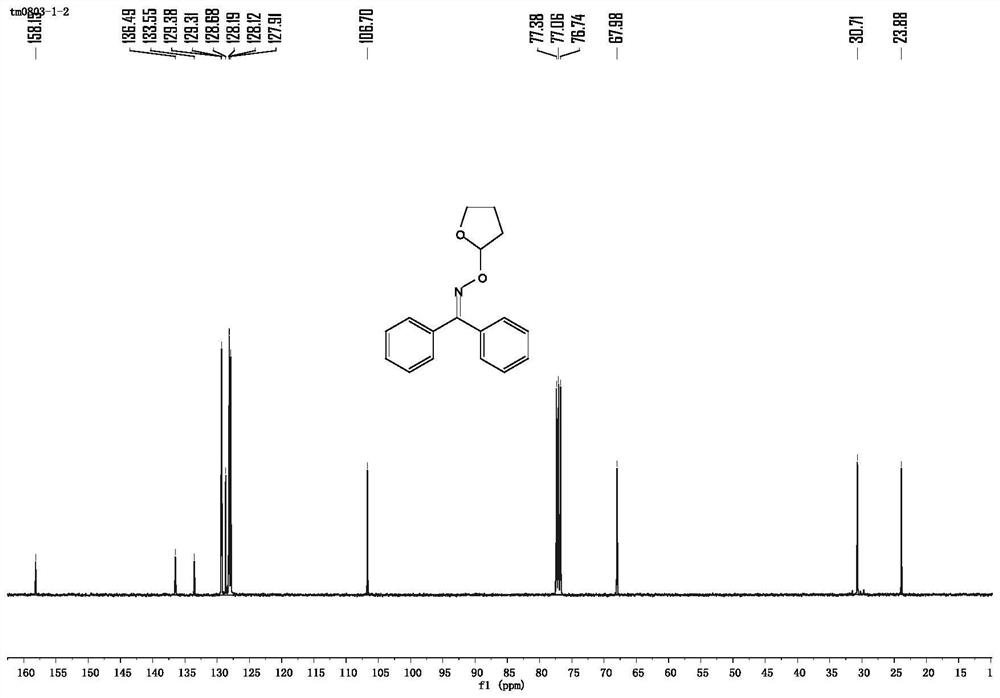
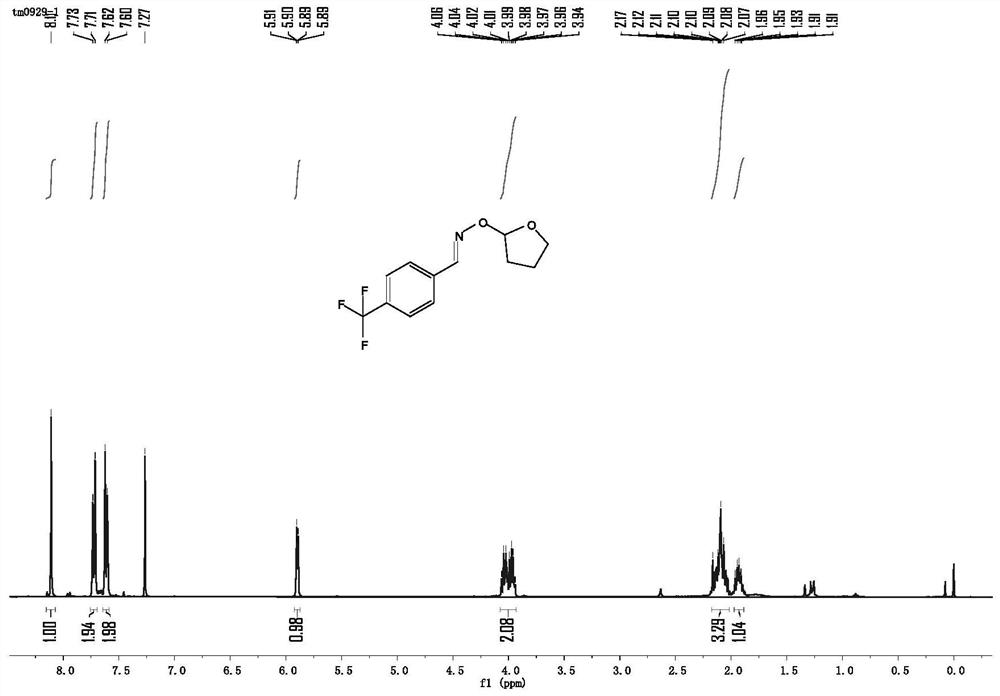
[0052] 0.0189g p-trifluoromethylbenzaldehyde oxime (0.1mmol) is replaced by 0.0197g benzophenone oxime (0.1mmol), other conditions and operating procedures are the same as in Example 1, and the obtained pure product p-trifluoromethylbenzaldehyde- O-2-Tetrahydrofuryl oxime ether in 90% yield. like image 3 shown: 1 H NMR (400MHz, CDCl 3 )δ8.11(s,1H),7.72(d,J=8.2Hz,2H),7.61(d,J=8.3Hz,2H),5.90(dd,J=5.0,1.5Hz,1H),4.07– 3.93 (m, 2H), 2.18–2.02 (m, 3H), 1.93 (dt, J=9.5, 4.6 Hz, 1H). like Figure 4 shown: 13 C NMR (101MHz, CDCl 3 )δ148.54(s), 135.59(s), 125.57(dd, J=7.5, 3.7Hz), 107.00(s), 77.36(s), 77.04(s), 76.73(s), 68.14(s), 30.82(s), 23.77(s).
Embodiment 3
[0054] 0.0149g 2,4-dimethylbenzaldehyde oxime (0.1mmol) was replaced by 0.0197g benzophenone oxime (0.1mmol), other conditions and operation process were the same as in Example 1, and the obtained pure product was 2,4-dibenzophenone oxime Methylbenzaldehyde-O-2-tetrahydrofuryl oxime ether in 93% yield. like Figure 5 shown: 1 H NMR (400MHz, CDCl 3 )δ8.32(s,1H),7.64(d,J=7.8Hz,1H),6.99(d,J=9.6Hz,2H),5.92-5.86(m,1H),4.08-3.91(m,2H ), 2.38 (s, 3H), 2.31 (s, 3H), 2.16–2.00 (m, 3H), 1.97–1.86 (m, 1H). like Image 6 shown: 13 C NMR (101MHz, CDCl 3 )δ148.92(s), 139.82(s), 136.75(s), 131.47(s), 127.52(s), 127.11(s), 127.00(d, J=20.8Hz), 106.58(s), 77.39( s), 77.07(s), 76.75(s), 68.00(s), 30.87(s), 23.89(s), 21.31(s), 19.73(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com