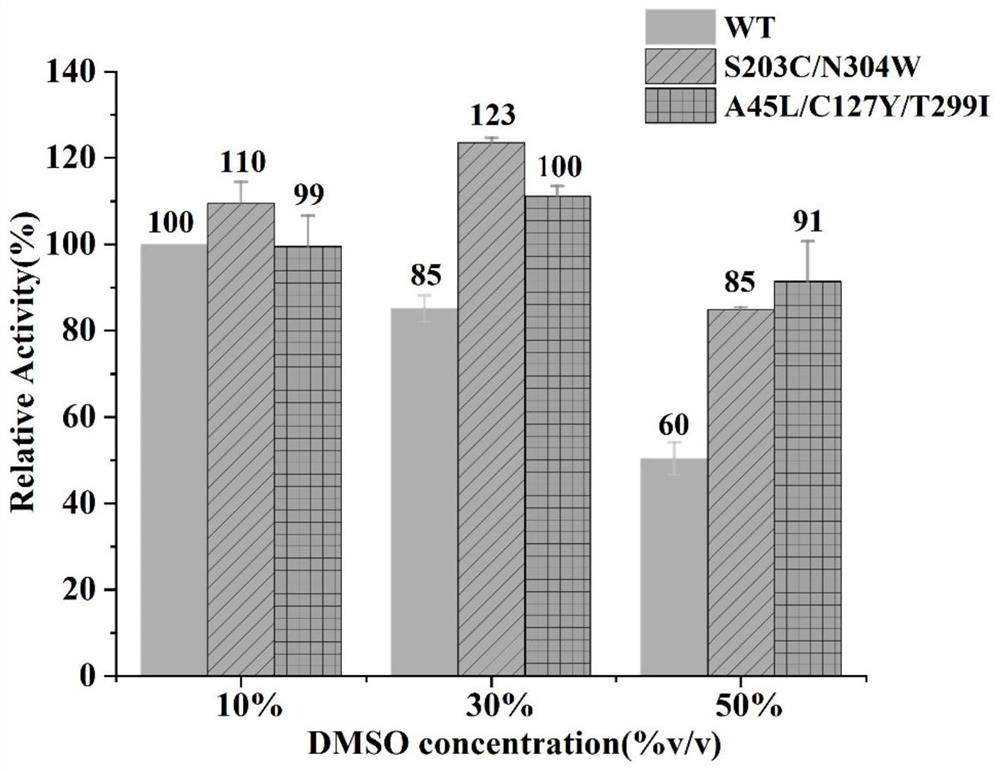
Glycosyl transferase mutant with improved thermal and organic solvent stability
A technology of glycosyltransferase and organic solvent, which is applied in the field of genetic engineering, can solve the problems of limited thermal stability and chemical stability of natural proteins, restricting the practical application of industrialization, etc., to improve the tolerance of organic solvents, enhance the application value, heat The effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
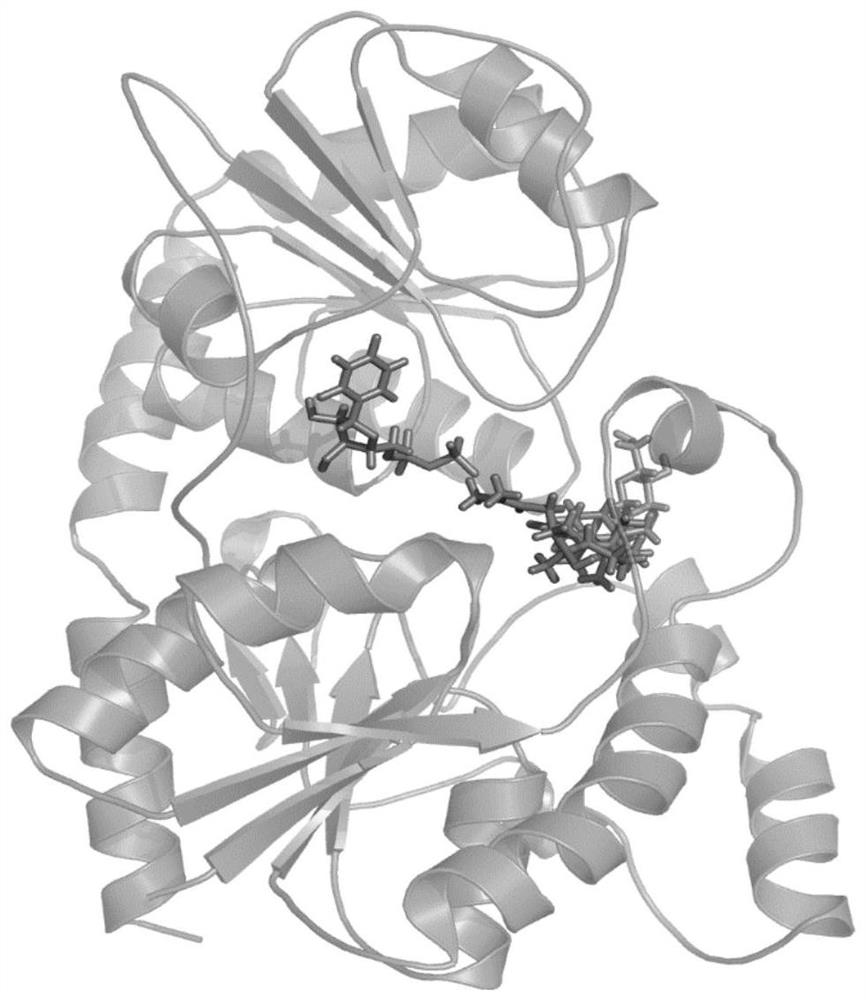
[0049] Purify the wild-type glycosyltransferase protein recombinantly expressed in E.coli BL21 (DE3) with a 6xHis tag at the N-terminus, and use the Hampton commercial protein crystallization kit to perform extensive protein crystallization conditions screening to obtain initial protein crystals and optimize crystal growth. The pH, precipitant, salt concentration and other conditions were used to obtain UGT109A3 enzyme and donor substrate UDP-glucose complex crystals suitable for diffraction. The data were collected by X-ray diffraction at the Shanghai Light Source (SSRF) BL 19U1 line station. The diffraction data processing online station HKL3000 software is integrated to obtain the scale suffix file, and the resolution reaches The crystal structure of glycosyltransferase UGT109A3-UDP complex was solved by molecular replacement; the structure of UGT109A3-UDP is a typical GT-B fold, consisting of N-terminal domain (NTD) and C-terminal domain (C -terminal domain, CTD) consists...
Embodiment 2
[0070] Heterologous expression and purification of glycosyltransferase UGT109A3 mutant
[0071] Specific steps are as follows:
[0072] The transformants with correct sequencing were picked and transferred to LB liquid medium containing kanamycin (50 mg·L-1), cultured at 37°C and 220 rpm / min, and the overnight culture medium was inoculated with kanamycin-containing medium at a ratio of 1%. of autoinduction medium, cultivated to OD at 37°C at 220rpm 600 About 0.6-0.8, add isopropyl 1-β-D-thiogalactopyranoside (IPTG to the medium, the final concentration is 0.5mM L -1 )), and the induction culture was continued for 20 hours at 18°C.
[0073] The cultured bacterial solution was collected by centrifugation at 5000rpm for 15min at 4°C, and 10mL bindingbuffer (pH 8.0, 50mM Tris-HCl buffer, 500mM NaCl, 20mM imidazole) was added to the ratio of 1g wet bacteria to the bindingbuffer, resuspended at high pressure Broken in a homogenizer. The crushed solution was centrifuged at 4°C an...
Embodiment 3
[0075] Determination of Tm Value of Glycosyltransferase UGT109A3 Mutant
[0076] Specific steps are as follows:
[0077] Combine UGT109A3 glycosyltransferase mutant protein with SYPRO TM Orange fluorescent dye was combined, and the melting curve was measured in a real-time quantitative PCR instrument (Real-time qPCR). The reaction system is shown in Table 2.
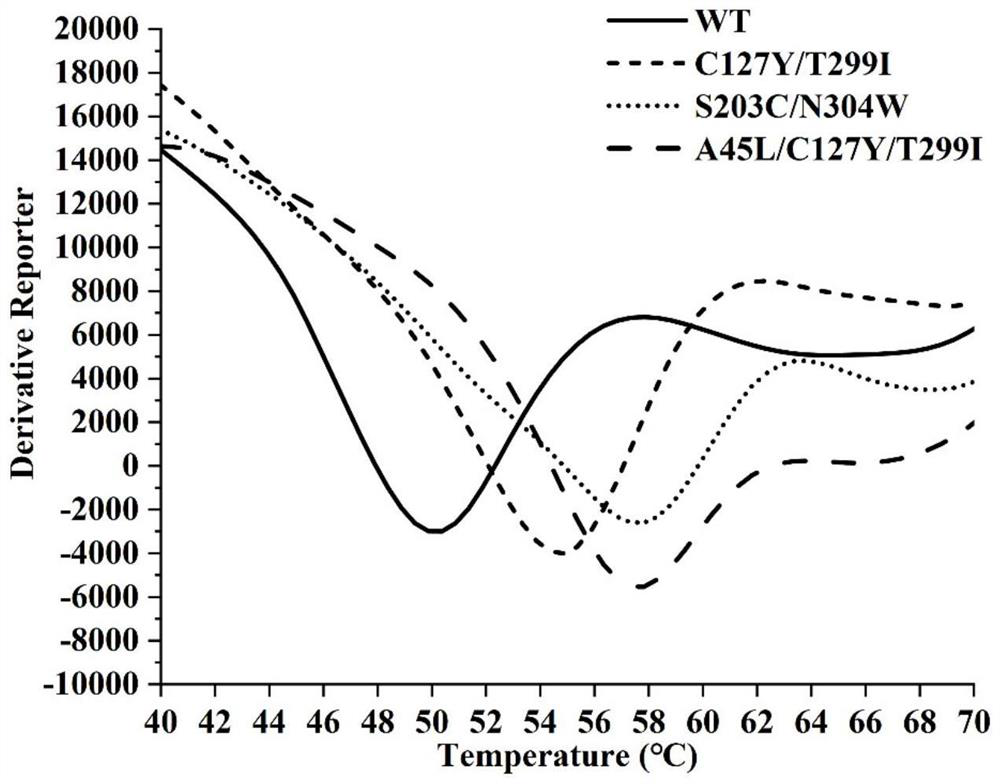
[0078] Combining glycosyltransferase UGT109A3 mutant protein with SYPRO TM After the Orange protein gel stain was thoroughly mixed in the eight-connected tube, the eight-connected tube was placed in a fluorescence quantitative PCR instrument for measurement. Set the relevant measurement program, gradually increase the temperature from 25°C to 90°C, obtain the melting curve, and calculate the Tm value. The melting curve results are as follows: figure 1 shown.
[0079] Using differential scanning calorimetry (Differential Scanning Calorimetry, DSC), it was confirmed that the obtained glycosyltransferase UGT109A3 double...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com