Poly acid anhydride for release-controlled medicine carrier and method for producing the same
A technology of polyanhydride and dimer acid, which is applied in the field of chemically synthesized drug sustained-release materials, to achieve good biocompatibility, long drug release period, and slow degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
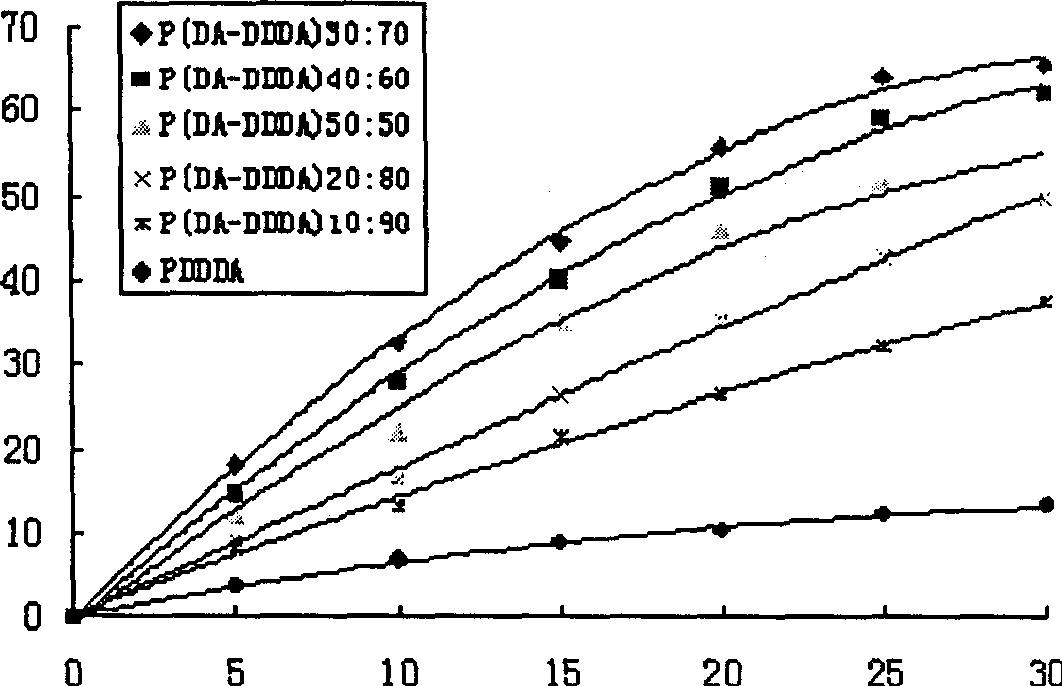
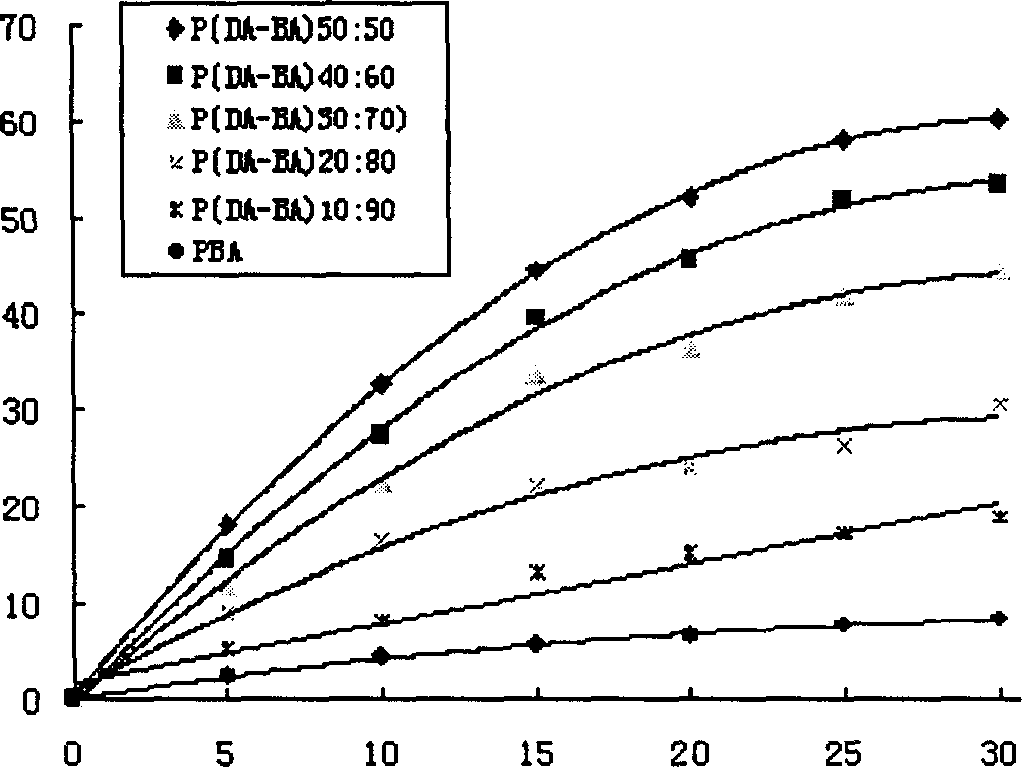
[0044] Example 1: Synthesis of poly(dimer acid-lauric acid) copolymer anhydride
[0045] Add 2 grams of lauric acid prepolymer and 2 grams of dimer acid prepolymer into a polymerization tube with a diameter of 2 cm and a length of 20 cm, place it in a silicone oil bath, and stir it electromagnetically. The temperature was raised to 165°C, the vacuum pressure was reduced to 40 Pa, the melt polymerization was carried out for 75 minutes, nitrogen gas was passed for 15 seconds every 15 minutes, and the vacuum was drawn again. After the polymerization is completed, cool down to obtain a pale yellow solid, which is dissolved in dichloromethane, filtered through a G4 sand core funnel, and petroleum ether is precipitated. The precipitate is washed with methyl ethyl ether and dried to obtain a slightly yellow powdery solid. The synthesis of other different prepolymer mass ratios is carried out similarly as above. The FT-IR and physical and chemical properties of 5 different proportion...
example 2
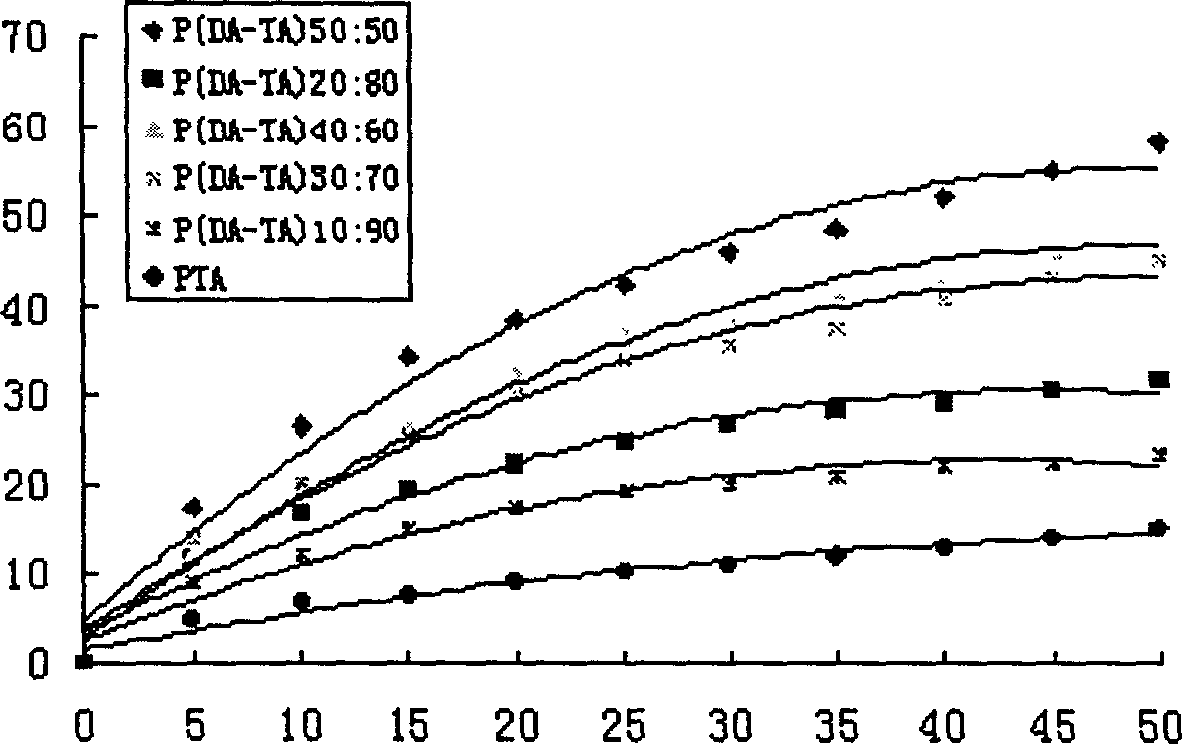
[0047] Example 2: Synthesis of poly(dimer acid-tetradecanedioic acid) copolymer anhydride
[0048] Add 2 grams of dimer acid prepolymer and 3 grams of tetradecanedioic acid prepolymer into a polymerization tube with a diameter of 2 cm and a length of 20 cm, place it in a silicone oil bath, and stir it electromagnetically. The temperature was raised to 170°C, the vacuum pressure was reduced to 45 Pa, the melt polymerization was carried out for 85 minutes, and nitrogen gas was flowed for 10 seconds every 10 minutes, and then vacuumed. After the polymerization is completed, cool down to obtain a pale yellow solid, which is dissolved in dichloromethane, filtered through a G4 sand core funnel, precipitated with cyclohexane, washed with anhydrous ether and dried to obtain a slightly yellow powdery solid. The synthesis of other different prepolymer mass ratios is carried out similarly as above. The FT-IR and physical and chemical properties of 5 different proportions of polyanhydrid...
example 3
[0050] Example 3: Sustained release characteristics of ciprofloxacin hydrochloride in poly(dimer acid-lauric acid) copolymer anhydride-ciprofloxacin hydrochloride sticks
[0051] Mix ciprofloxacin hydrochloride with polyanhydride, grind it in an agate mortar, heat the mixture to about 90°C with an infrared lamp to melt it, and use a self-made polytetrafluoroethylene mold to prepare a drug load of 20% cylindrical polyanhydride drug rod (φ4mm, length 10mm). The drug sticks were respectively placed in 50 mL of 0.1 mol / L, pH=7.4 phosphate buffer solution, and the degradation experiment was carried out on a constant temperature shaker at 37° C. (rotating speed 60 rad / min). The buffer solution is replaced at regular intervals, and the absorbance of the release medium at a wavelength of 271nm is measured by UV spectroscopy, based on the regression of the measured ciprofloxacin hydrochloride at 271nm in a 0.1mol / L, pH=7.4 phosphate buffer solution Equation A=0.27465ρ-5.133×10-4, wher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com