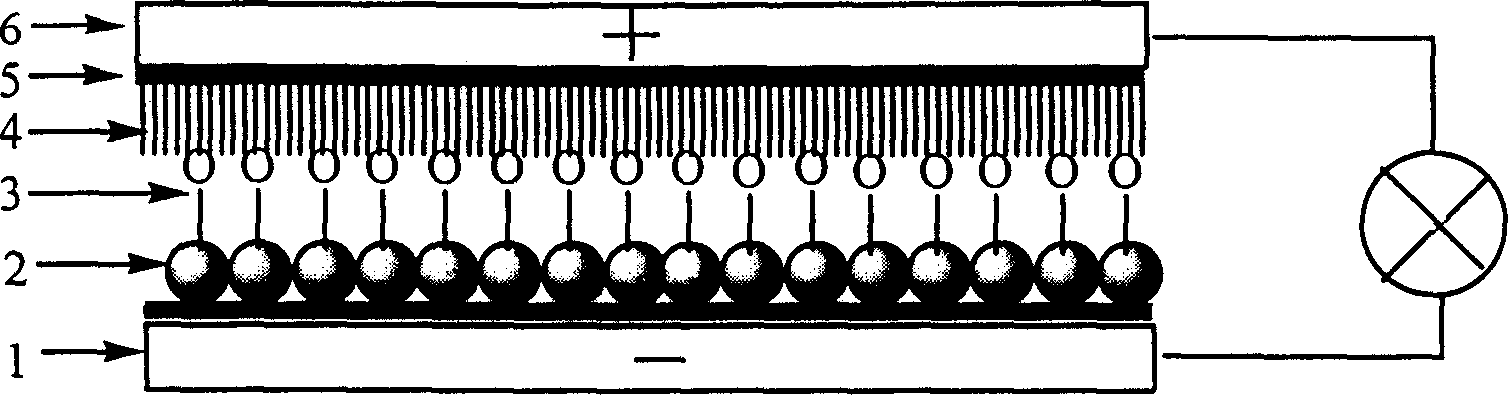
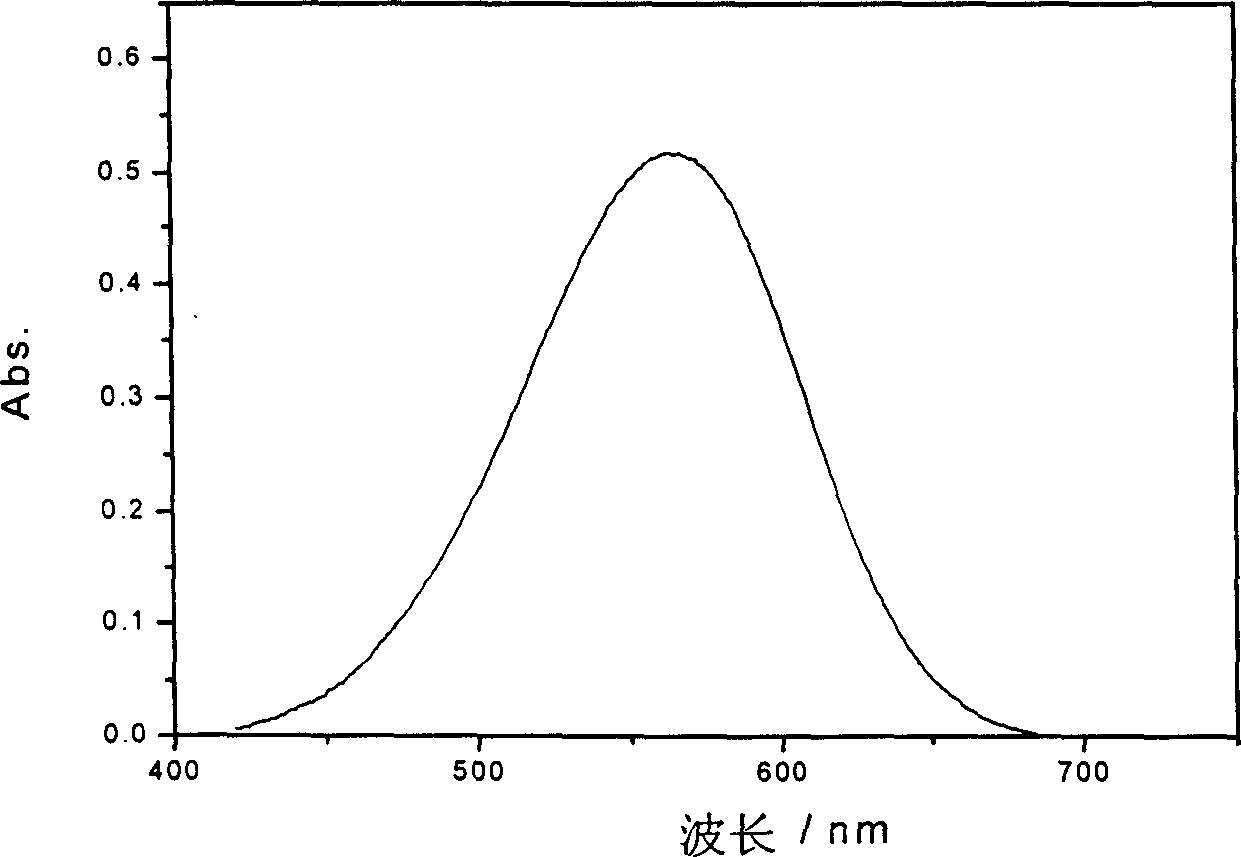
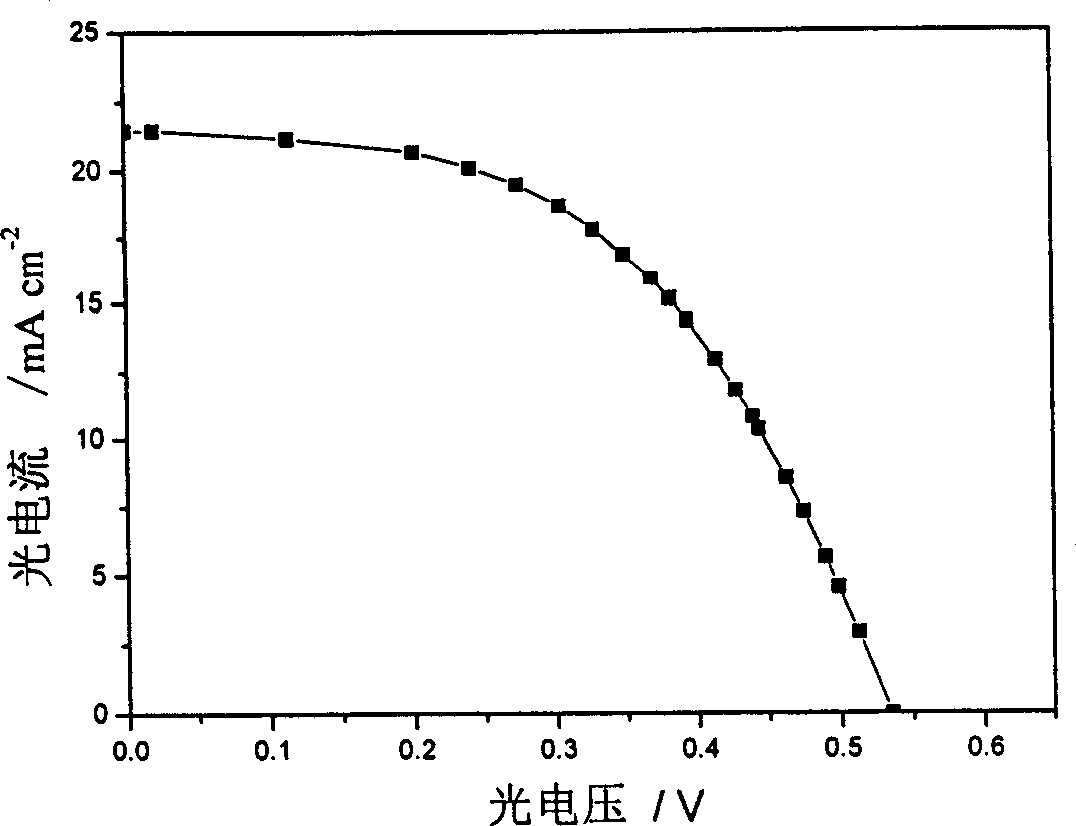
Large conjugated half cyanine dye, its synthesis and its sensitized nano-crystal semiconductor solar energy battery
A technology of bonding and compounding, applied in semiconductor devices, semiconductor/solid-state device manufacturing, circuits, etc., can solve the problem of insufficient capture ability and achieve good photoelectric conversion function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: N-(3-sulfonate propyl)-2-[2-(4-N,N-dimethylaminophenyl)vinyl]pyridine Onium Synthesis of Inner Salt (Dye-1)
[0083]
[0084] (1) Preparation of N-(3-sulfonate propyl)-2-picoline inner salt
[0085] 0.93g (0.01mol) of 2-picoline and 1.22g (0.01mol) of 1,3-propiolactone sulfonate were dissolved in 20mL of dry benzene and refluxed for 48h, then cooled at room temperature. The solid was collected by suction filtration and washed with anhydrous benzene to obtain 1.46 g of N-(3-sulfopropyl)-2-picoline inner salt as a white solid. Yield 68%.
[0086] (2) Synthesis of N-(3-sulfonate propyl group)-2-[2-(4-N, N-dimethylaminophenyl) vinyl] pyridinium inner salt
[0087] 0.215 g (0.001 mol) of the product of the above step was dissolved in 30 mL of absolute ethanol, and 0.149 g (0.001 mol) of 4-N, N-dimethylaminobenzaldehyde and a drop of hexahydropyridine were added. The reaction mixture was refluxed for 16h with stirring. Cool to room temperature. The reac...
Embodiment 2
[0091] Example 2: N-(3-sulfonate propyl)-2-[2-(4-N,N-diethylaminophenyl)vinyl]pyridine Synthesis of Inner Salt (Dye-2)
[0092]
[0093] Synthesize according to the method described in Example 1, just replace 4-N, N-dimethylaminobenzaldehyde with 4-N, N-diethylaminobenzaldehyde. After recrystallization from ethanol, 0.285 g of the target compound was obtained as orange-red crystals, with a yield of 76%. m.p.258~9℃.
[0094] Elemental Analysis C 20 h 26 N 2 o 3 S: Calculated: C, 64.14; H, 7.00; N, 7.48. Found: C, 63.96; H, 6.94; N, 7.16.
[0095] 1 H(500MHz, DMSO) δ: 1.14(t, 6H, J=6.90Hz) 2.16(m, 2H) 2.60(t, 2H, J=6.17Hz) 3.45(q, 4H, J=6.90Hz) 4.85(t , 2H, J = 6.93Hz) 6.74 (d, 2H, J = 8.71Hz) 7.45 (d, 1H, J = 15.56Hz) 7.69 (t, 1H, J = 6.61Hz) 7.83 (d, 2H, J = 8.71 Hz) 7.96 (d, 1H, J = 15.51 Hz) 8.30 (t, 1H, J = 7.88 Hz) 8.49 (d, 1H, J = 8.50 Hz) 8.74 (d, 1H, J = 6.27 Hz).
[0096] IR (cm -1 ): 2973, 1586, 1555, 1523, 1436, 1407, 1334, 1273, 1193, 1152, 1038, 81...
Embodiment 3
[0097] Example 3: 1-(3-sulfopropyl)-4-[2-(4-N,N-dimethylaminophenyl)vinyl]pyrimidine Synthesis of Salt (Dye-3)
[0098]
[0099] Synthesized according to the method described in Example 1, except that 2-methylpyridine was replaced by 2-methylpyrimidine. After purification by column chromatography (silica gel column, methanol:chloroform=1:8), 0.215 g of the target compound was obtained as a purple solid, with a yield of 62%. m.p.281~2℃.
[0100] Elemental Analysis C 17 h 21 N 3 o 3 S: Calculated: C, 58.77; H, 6.09; N, 12.09. Found: C, 58.71; H, 6.32; N, 11.96.
[0101] 1 H(500MHz, DMSO) δ: 2.19~2.24(m, 2H) 2.48(t, 2H, J=6.11Hz) 3.07(s, 6H) 4.48(t, 2H, J=6.95Hz) 6.82(d, 2H, J = 8.87Hz) 7.23 (d, 1H, J = 15.48Hz) 7.72 (d, 2H, J = 8.83Hz) 7.91 (d, 1H, J = 6.81Hz) 8.26 (d, 1H, J = 15.48Hz) 8.93 (dd, 1H, J = 6.84, 1.30 Hz) 9.37 (s, 1H).
[0102] IR (cm -1 ): 3048, 2917, 1577, 1530, 1451, 1375, 1337, 1293, 1162, 1041, 942, 826, 731, 524.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com