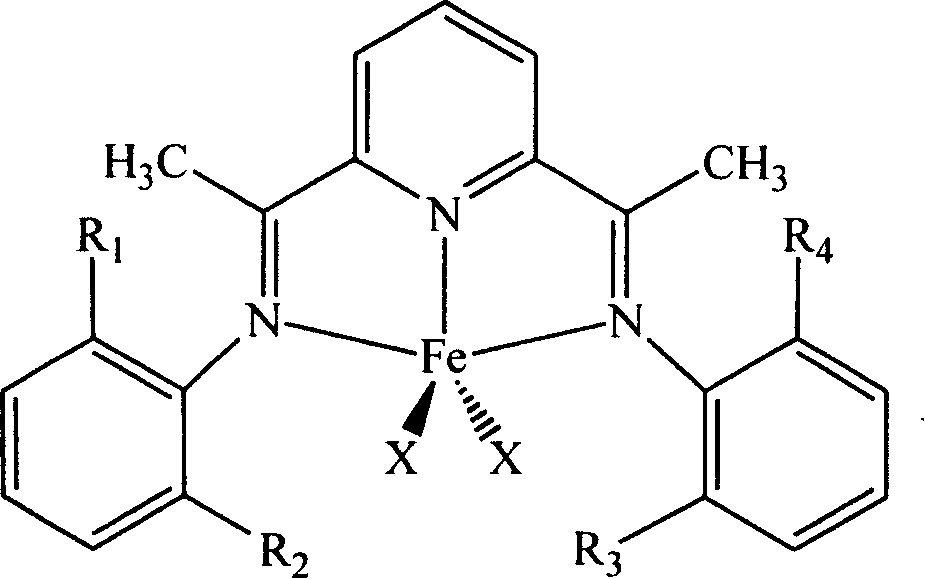
Carried catalyzer for olefinic polymerization and preparation method
An olefin polymerization and catalyst technology is applied in the field of supported olefin polymerization catalyst and its preparation, and achieves the effects of excellent ion exchange performance, high catalytic activity and large pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of the catalyst: Add 1.0000g (6.13mmol) of 2,6-diacetylpyridine into a 100mL Schlenk flask equipped with a stirring magnet, and inject 14.9196g into the reactor equipped with a stirring magnet with a syringe under the protection of argon. (24.52mmol, 4equiv.) 2,6-Diisopropylaniline, 80mL anhydrous methanol and 0.2mL formic acid, react at 60°C for 48h. Cool, filter, and wash with cold methanol to obtain a yellow powdery product. After drying, it was extracted with isopropanol for 24 hours to remove the monoimine by-product, and a pale yellow powdered ligand was obtained. Weigh a certain amount of ligand (1.05equiv.) into a 20mL Schlenk bottle equipped with a magnetic stirrer, and add FeCl 2 ·4H 2 O(1.00equiv.). Evacuate and ventilate with argon three times, inject 10 mL of dry tetrahydrofuran under the protection of argon, and react at 25°C for 2h. After the reaction, 5 mL of n-pentane was added, then filtered, washed with n-pentane and anhydrous ether, and dried...
Embodiment 2
[0034] Preparation of the catalyst: the same as in Example 1.
[0035] Preparation of carrier precursor: CaO, SiO 2 , K 2 O, H2 O was put into a stainless steel reactor at a weight ratio of 1:1:1.5:1000, and crystallized at 500°C for 25 days to obtain a carrier precursor molecular sieve calcium silicon compound.
[0036] Preparation of supported catalyst: After 5g of molecular sieve calcium silicon compound was vacuum dried at 30°C for 10 hours, 20mL of 1.8mol / L triethylaluminum toluene solution was added, stirred at 50°C for 6 hours, filtered, and heated with anhydrous toluene Washing and filtering, 20mL each time, washing three times in total, after filtering and drying to prepare molecular sieve-like calcium silicate compound carrier. 0.25 g of catalyst Ia was added to the above system and stirred at 40° C. for 10 hours. Wash with toluene several times until the supernatant becomes colorless. Vacuum dry and store under inert gas for later use.
[0037] Ethylene polymerization: ...
Embodiment 3
[0039] Preparation of the catalyst: Add 1.0000g (6.13mmol) of 2,6-diacetylpyridine to a 100mL Schlenk flask equipped with a stirring magnet, and inject 24.3361g (49.04) into a device equipped with a stirring magnet under the protection of argon. mmol, 8 equiv.) 2,6-dimethylaniline, 80 mL anhydrous methanol and 0.2 mL formic acid, react at 60° C. for 48 h. Cool, filter, and wash with cold methanol to obtain a yellow powdery product. After drying, it was extracted with isopropanol for 24 hours to remove the diimine by-products to obtain a pale yellow powdery intermediate. Weigh a certain amount of ligand (1.05equiv.) into a 20mL Schlenk bottle equipped with a magnetic stirrer, and add FeCl 2 ·4H 2 O(1.00equiv.). Evacuate and ventilate with argon three times, inject 10 mL of dry tetrahydrofuran under the protection of argon, and react at 25°C for 2 h. After the reaction, 5 mL of n-pentane was added, then filtered, washed with n-pentane and anhydrous ether, and dried under vacuum at r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com