Method for improving polarity of flavonoid glycoside
A flavonoid aglycone and polarity technology, which is applied in the field of improving the polarity of compounds, can solve the problems of high production cost, large amount of ethanol, low polarity of quercetin, etc., so as to improve the extraction rate, reduce the amount of ethanol and expand the practical application. range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, improve the polarity of quercetin
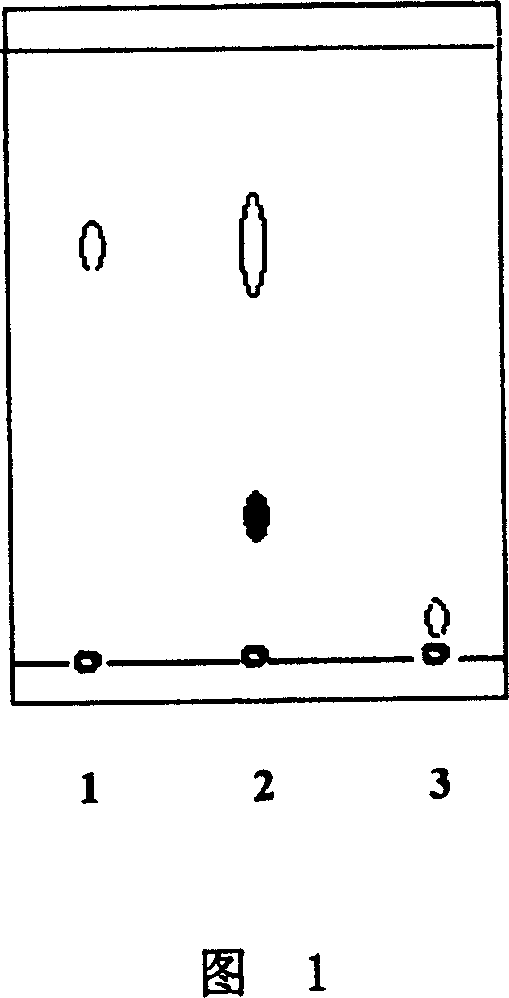
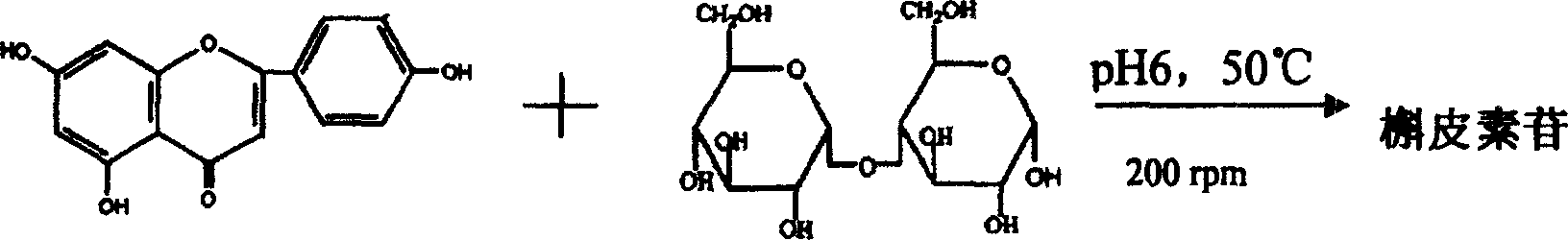
[0024] In a 1000mL conical flask, add 0.025g quercetin, 0.1125g maltose and 50mL 30% ethanol aqueous solution, adjust the pH value to 6.0 with 1N NaOH solution, then add 0.5mL of penicillium decumbens glucosidase solution (6.23IU / mL), Finally, the reaction was stirred for 30 hours at 50° C. and 200 rpm. After the reaction, the reaction product was extracted with the same volume of ethyl acetate (with rutin as a positive control), and the extract was prepared on a GF254 silica gel plate (10cm×20cm), benzene:ethyl acetate:acetone:acetic acid=10:8:2 : 2 is mobile phase development, observes fluorescence under 365nm ultraviolet lamp and carries out qualitative and quantitative analysis with TLC method, the result is as shown in Figure 1 (1.R f槲皮素 =0.68,
[0025] 2. R f槲皮素转苷产物 =0.30, 3.R f芦丁 =0.01), it shows that the quercetin glycosides whose polarity is greater than quercetin have been produced by the transglycoside rea...
Embodiment 2
[0027] Embodiment 2, improve the polarity of kaempferol
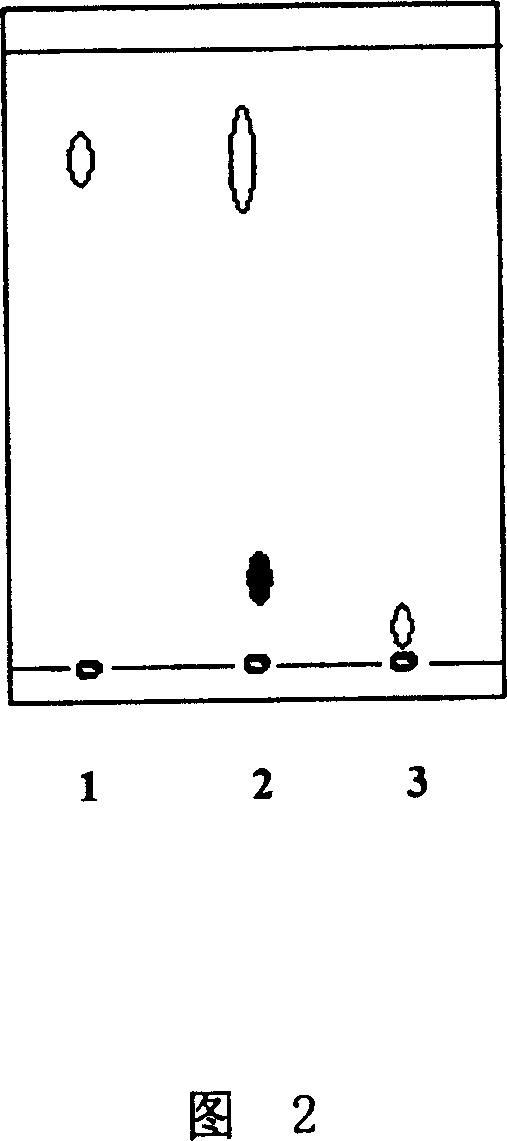
[0028] In a 1000mL conical flask, add 0.015g kaempferol, 0.072g maltose and 50mL 60% ethanol aqueous solution, adjust the pH value to 9.0 with 1N NaOH solution, then add 2.0mL of spirizyme plus glucosidase (standard activity 400AGU / g), The reaction was stirred for 1 hour at 30°C, 200 rpm. After the reaction was finished, extract with the same volume of ethyl acetate (with rutin as a positive control), and extract the solution on a GF254 silica gel plate (10cm×20cm), benzene: ethyl acetate: acetone: acetic acid = 10:8:2:2 For mobile phase development, observe fluorescence under 365nm ultraviolet lamp and carry out qualitative and quantitative analysis with TLC method, the result is as shown in Figure 2 (1.R f山萘酚 =0.87, 2.R f山萘酚转苷产物 =0.10, 3.R f芦丁 =0.01), it shows that the kaempferol glycosides with greater polarity than kaempferol have been produced after the glucoside conversion reaction, so that it is easily dissolv...
Embodiment 3
[0030] Embodiment 3, improve the polarity of quercetin
[0031] In the 1000mL conical flask, add 0.025g quercetin, 0.125g maltose and 50mL 30% ethanol aqueous solution, adjust the pH value to 2.0 with 1N NaOH solution, then add suhong475 glucosidase (standard activity 475AGU / g) 0.5mL, in Stir the reaction at 90° C. and 200 rpm for 30 hours. After the reaction, extract with the same volume of ethyl acetate (with rutin as a positive control), the extract was prepared on a GF254 silica gel plate (10cm×20cm), benzene:ethyl acetate:acetone:acetic acid=10:8:2:2 The mobile phase is developed, the fluorescence is observed under a 365nm ultraviolet lamp and the qualitative and quantitative analysis is carried out with the TLC method, the results are as shown in Figure 1 (1.R f槲皮素 =0.68, 2.R f槲皮素转苷产物 =0.30, 3.R f芦丁 =0.01), it shows that quercetin glycosides with a greater polarity than quercetin have been produced through the glucoside conversion reaction, making it easy to be dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com