Metal chelate-forming fiber, process for producing the same, method of trapping metal ion with the fiber, and metal chelate fiber
A technology of metal chelation and synthetic fiber, which is applied in the ion exchange of chelate, synthetic fiber, ion exchange, etc., and can solve the problems of complex modification and treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
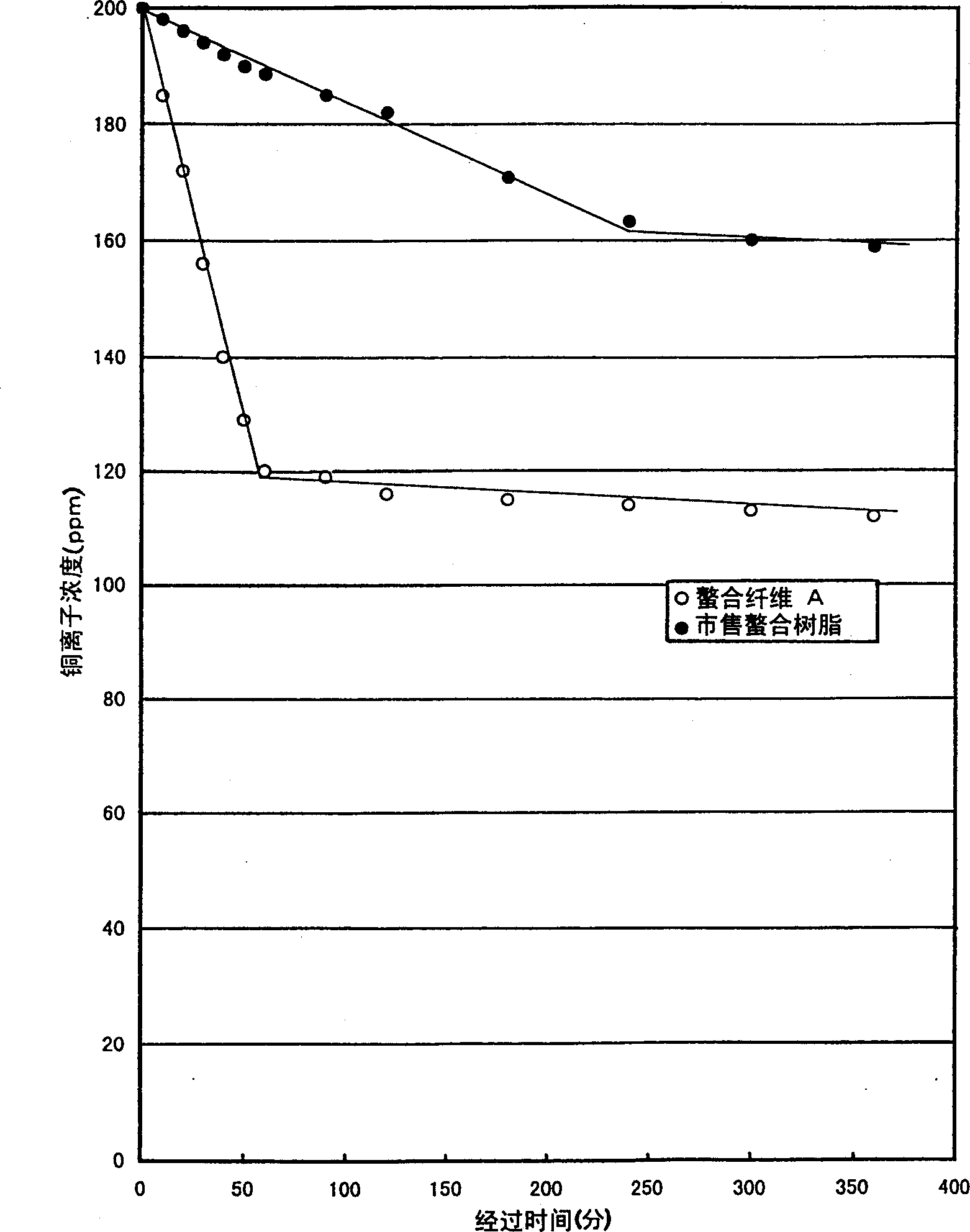
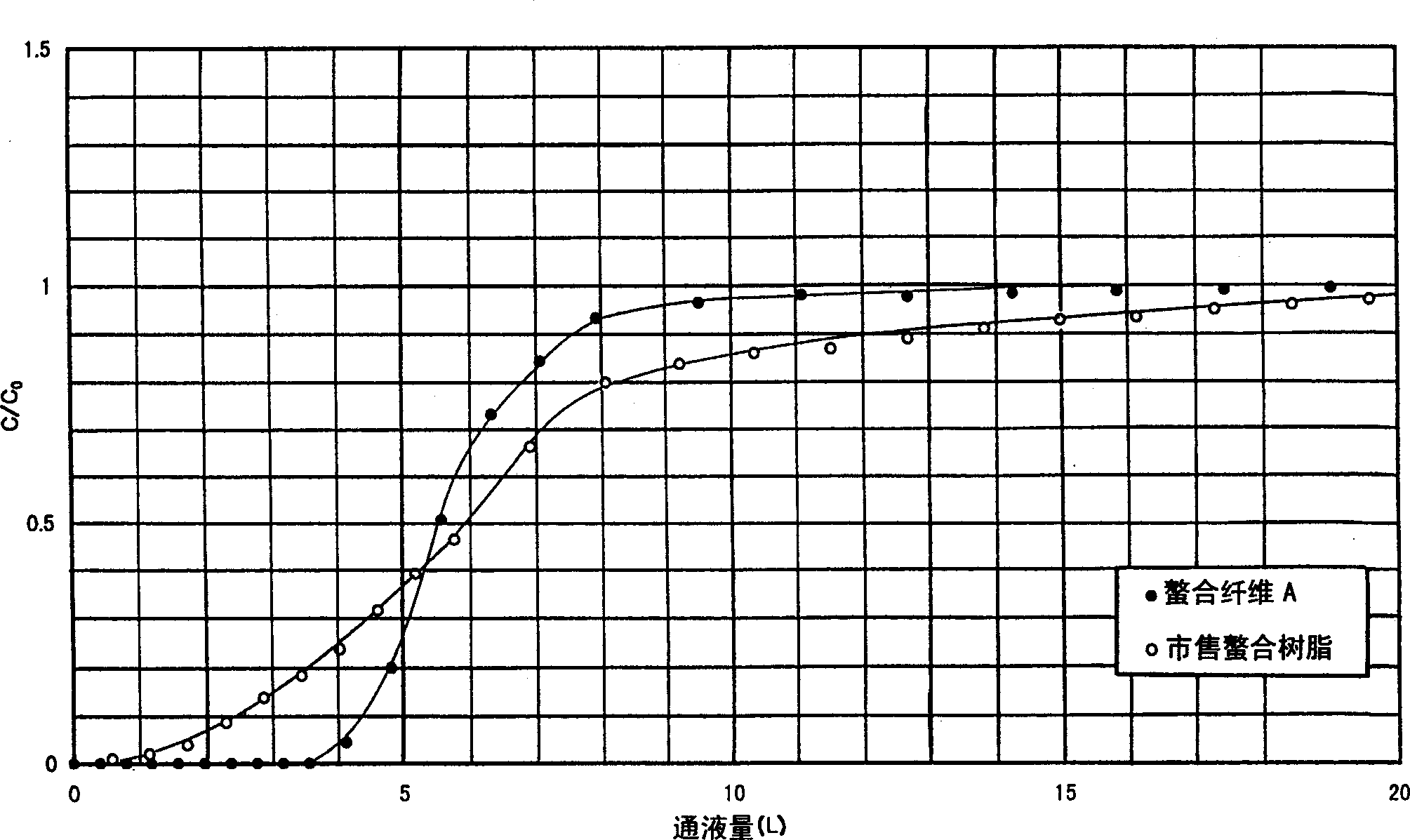
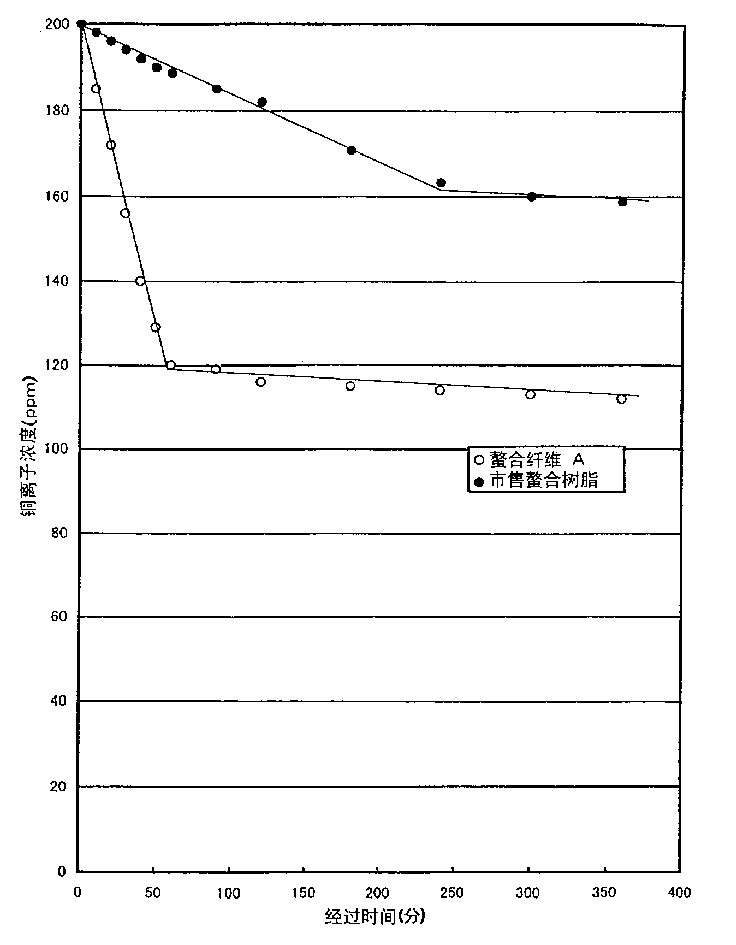
[0072] In a solution in which 5 g of potassium peroxodisulfate was dissolved in 250 mL of distilled water, 2.5 g of nylon 6 fabric was immersed at 20°C for 30 minutes, then centrifuged to remove the liquid, and added to a solution in which 250 mL of distilled water and 1.5 g of glycidyl methacrylate were dissolved. g and a solution of 0.5 g of copper sulfate 5 hydrate, and treated at 80° C. for 30 minutes. Then, the treated nylon fabric was washed with distilled water, centrifuged and dried for 16 hours to obtain 3.5 g of a nylon 6 fabric grafted with glycidyl methacrylate.
[0073] Then, 20 g of iminodiacetic acid was added to 100 g of distilled water, the pH was adjusted to 10 with a 50% aqueous sodium hydroxide solution, the grafted fabric was immersed in this solution, and treated at 90° C. for 2 hours. Then, after sufficient water eluent, 100 mL of 5% sulfuric acid was washed at 20°C for 30 minutes, washed with water, centrifuged and dried at 50°C for 16 hours to obtain m...
Embodiment 2
[0083] A commercially available cartridge filter (manufactured by Rokitechno, trade name "Micro-silia Filter Cartridge 250 LNS-1": nominal pore size 1 μm) in which nylon spun yarns were interlaced and wound on a polypropylene core material was mounted on the In a polypropylene cavity (manufactured by Advantec Toyo Co., Ltd., trade name "IPP-1-FS000"), a solution of 200 g of potassium peroxodisulfate dissolved in 10 liters of distilled water was passed through it at a flow rate of 15 liters / min for 20 °C × 15 minutes after circulation, the liquid was drained, and 5 liters of distilled water was also circulated for cleaning.
[0084] Then, a solution in which 60 g of glycidyl methacrylate and 10 g of sulfuric acid ketone 5 water and 10 g were dissolved in 8 liters of distilled water was also circulated at 80 ° C for 2 hours, and then glycidyl methacrylate and nylon spun yarn molecules were grafted After that, the reaction solution was discharged, and 3 liters of distilled water ...
Embodiment 3
[0089] In addition to replacing the iminodiacetic acid aqueous solution in Example 1 and using 30% ethylenediaminetriacetic acid 3 sodium salt aqueous solution 200g, it is the same as Example 1 to obtain 4.78g of metal chelate fiber (chelate fiber C) [Substitution rate: 88% (mass)]. Using this chelate fiber C, the same adsorption experiment as in Example 1 was performed, and it was confirmed that the copper capture ability of 1.0 mmoL was exhibited per 1 g of the chelate fiber.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com