Conjugates of phytosterol or phytostanol with ascorbic acid and use thereof in treating or preventing cardiovascular disease
A technology of plant stanols and plant sterols, applied in the application field of treating and preventing cardiovascular diseases and other diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
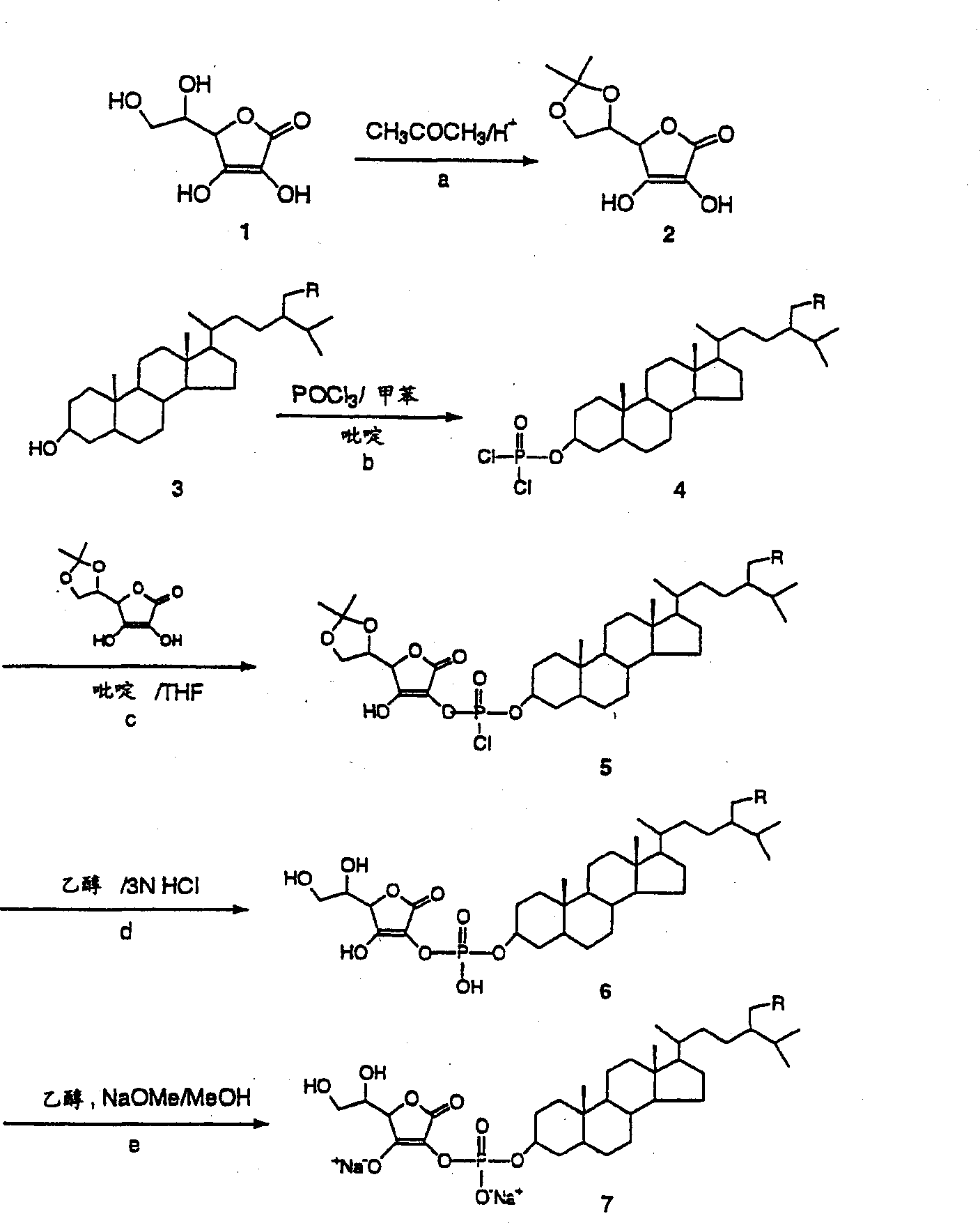
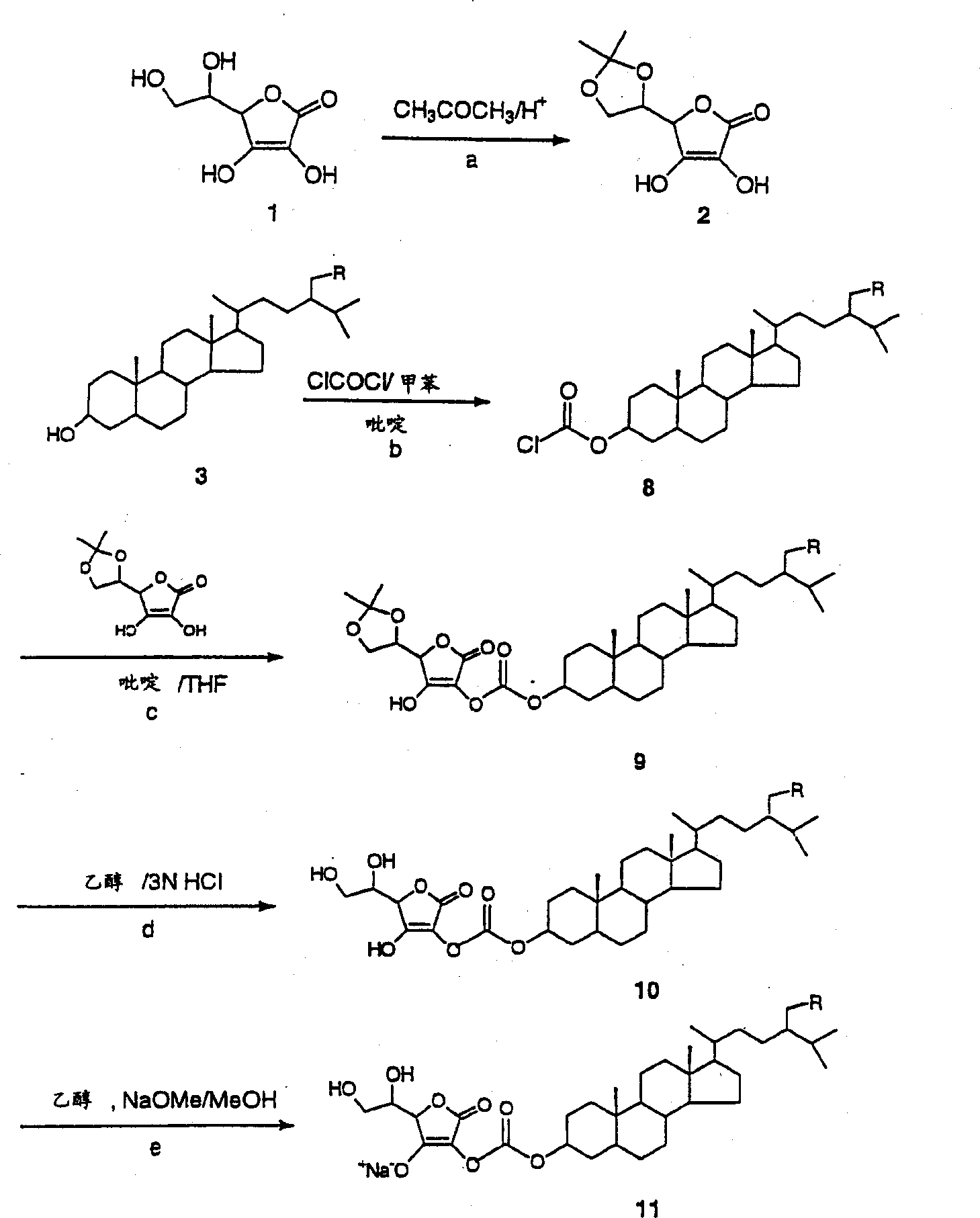
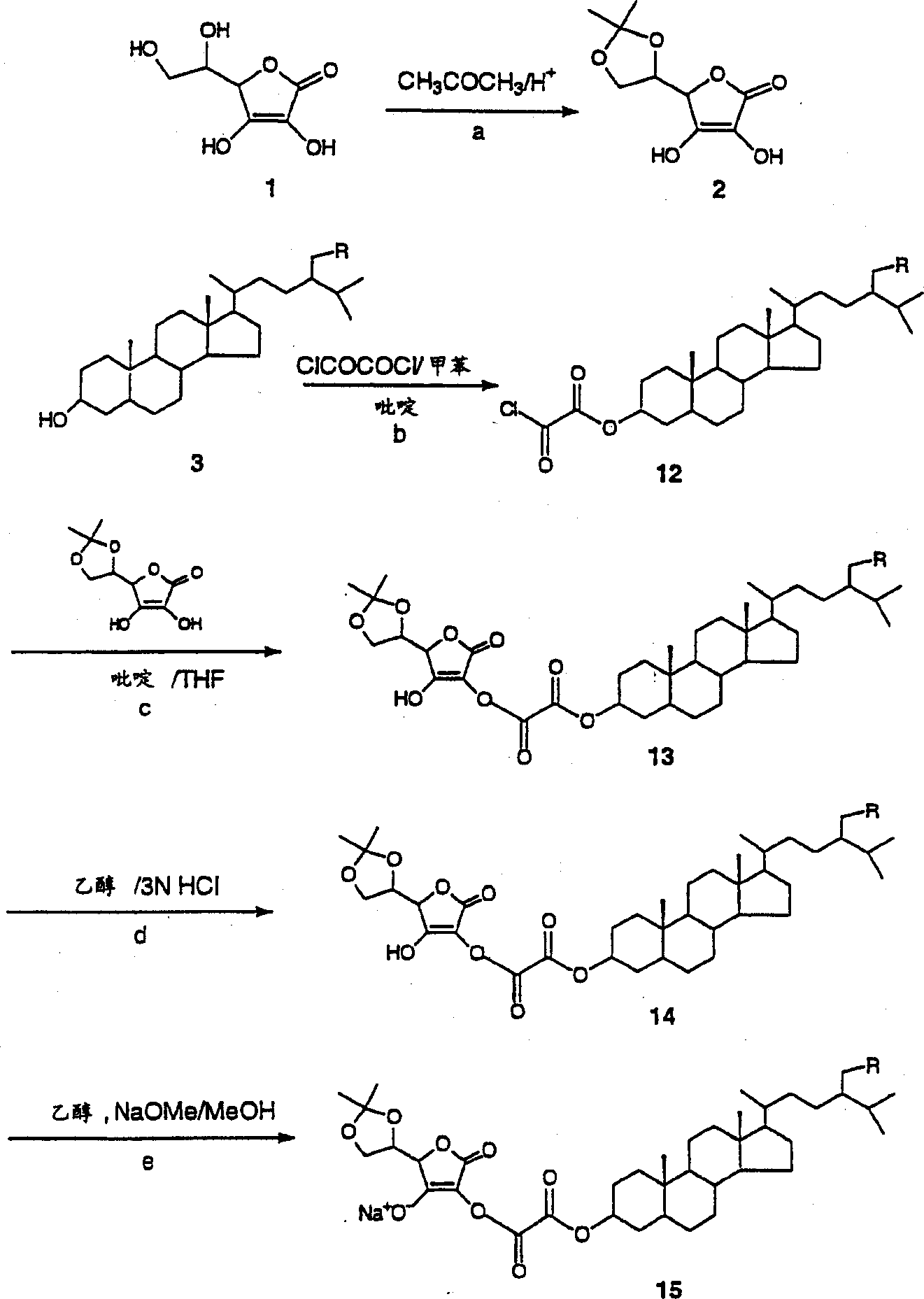
[0130] Example 1 - Protection of Ascorbic Acid
[0131] Fuming sulfuric acid (24%, 8.3 g) was added dropwise to acetone (50 mL). Ascorbic acid (12 g) was added to the mixture at 0°C, and the reaction mixture was stirred at 0°C for 6 hours. The resulting crystals were filtered off with suction, the filter cake was pressed dry and washed with acetone (30 ml). The product was obtained as 5,6-isopropylidene-ascorbic acid (14 g).
Embodiment 2
[0132] Example 2 - Linkage of Phytostanols
[0133] A solution of toluene (500 ml) and pyridine (25 ml) containing phytostanol mixture (24 g) (camesteranol: 36.4%; sitostanol: 62.3%) was added dropwise to 0°C containing phosphorus oxychloride ( 9 ml) in a mixture of toluene (200 ml). The mixture was stirred at room temperature for 3 hours. Pyridine hydrochloride was removed by filtration, the mother liquor was concentrated, and toluene was recovered. The residue was dissolved in dry THF (100 mL), and a solution of the above-prepared protected ascorbic acid (14 g) in dry THF (400 mL) was added at 0°C. Stirring was maintained at room temperature for 1 hour. The solution was concentrated and the solvent was removed. Ethanol (400 mL) and 3N HCl (200 mL) were added, the mixture was heated at 50°C for 30 minutes and concentrated. Ethyl acetate (600 mL) was added and the resulting solution was washed with water (3 x 300 mL), dried over sodium sulfate and concentrated to give the...
Embodiment 3
[0134] Example 3 - Conversion to sodium salt
[0135] The above-prepared acid (17 g) was dissolved in ethanol (100 ml), and a solution of sodium methoxide (2.7 g) in ethanol (50 ml) was added with stirring at room temperature. Stirring was maintained for 30 minutes. After the addition, the obtained white filter cake was filtered out, dried and weighed to obtain 20 g of white powder (phytostanol-phosphate-sodium ascorbate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com