Method for preparing nano zinc oxide
A nano-sized, zinc oxide technology, applied in chemical instruments and methods, zinc oxide/zinc hydroxide, chemical methods of reacting liquids with liquids, etc., can solve the problems of wide grain size distribution, difficult to control, etc. The effect of easy control of conditions and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step A: 0.12mol (34.5g) of solid ZnSO 4 ·7H 2 O dissolved in 120ml of H 2 In O; In addition, the solid NaOH of 0.15mol (6.0g) and the solid NaOH of 0.06mol (6.4g) 2 CO 3 Dissolve in 120ml of water.
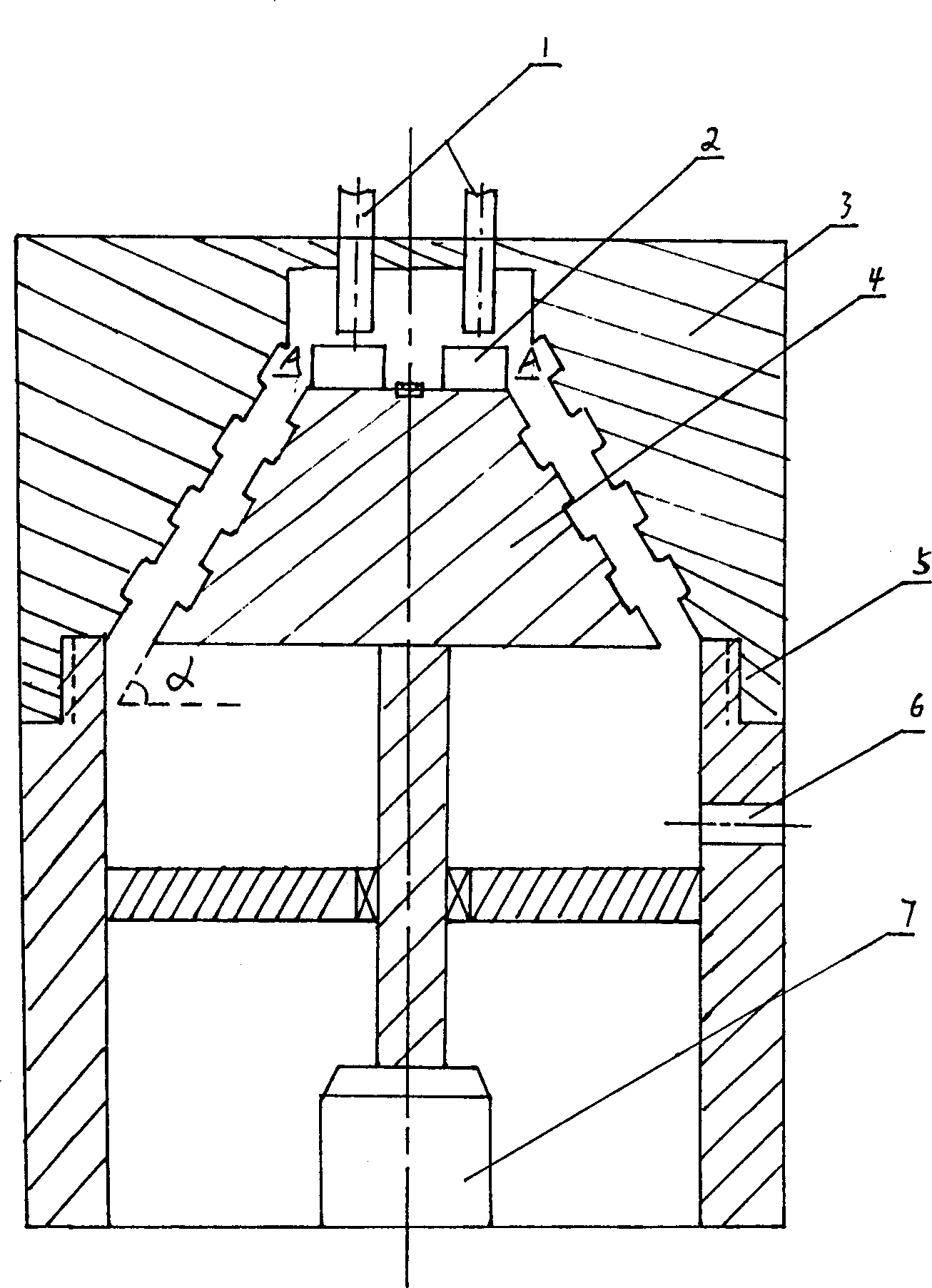
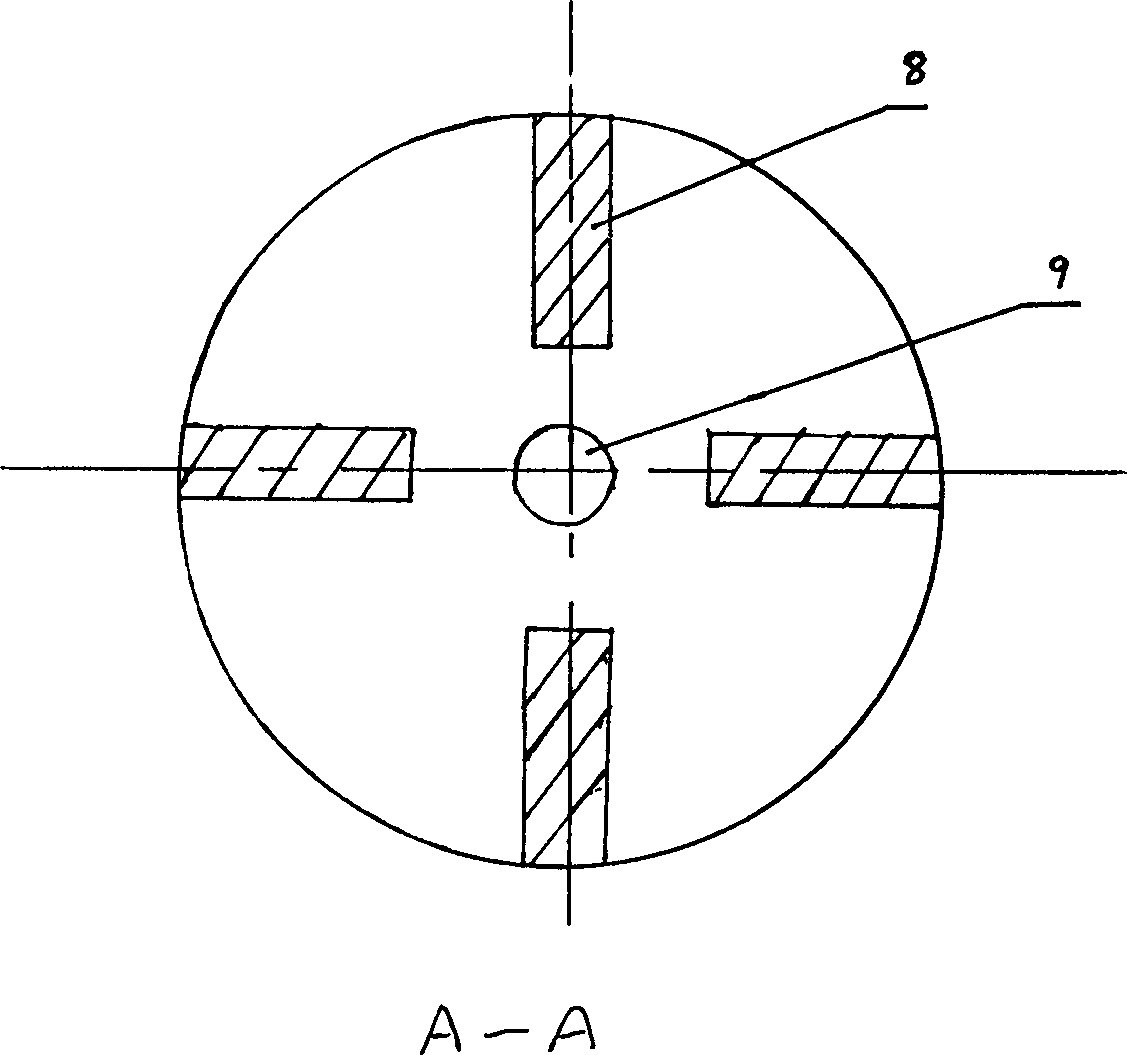
[0027] Step B: Add 80ml of deionized water into a fully back-mixed liquid membrane reactor with a volume of 1L, adjust the gap of the reactor to 10 microns, start the reactor, and control the rotor speed at about 6000rpm. Add the zinc salt solution and the alkali solution into the reactor at the same time, and the reaction mixture stays in the reactor for 2 minutes.
[0028] Step C: Pour the synthetic reaction material flowing out of the reactor in step B into a 500ml crystallization kettle, and crystallize at room temperature for 0.7h under stirring conditions.
[0029] Step D: The crystallized substance is filtered, washed and dried to obtain basic zinc carbonate.
[0030] Step E: Calcining basic zinc carbonate in a high-temperature furnace at 500°C to obtain zinc o...
Embodiment 2
[0033] Step A: 0.012mol (3.57g) of solid ZnNO 3 ·6H 2 O dissolved in 120ml of H 2 In O; another 25% NH 3 ·H 2 O 2ml and 0.024mol (2.3g) of solid [NH 4 ] 2 CO 3 Dissolve in 120ml of water.
[0034] Step B: Add 80ml of deionized water into a fully back-mixed liquid membrane reactor with a volume of 1L, adjust the gap of the reactor to 20 microns, start the reactor, and control the flow rate of the rotor at about 3000rpm. Add the zinc salt solution and the alkali solution into the reactor at the same time, and the reaction mixture stays in the reactor for 10 minutes.
[0035] Step C: Pour the synthetic reaction material flowing out of the reactor in Step B into a 500ml crystallization kettle, and crystallize at 5°C for 1 hour.
[0036] Step D: The crystallized substance is filtered, washed and dried to obtain basic zinc carbonate.
[0037] Step E: Calcining basic zinc carbonate at 300°C in a high-temperature furnace to obtain zinc oxide.
[0038] It can be seen from the...
Embodiment 3
[0040] Step A: 0.36mol (103.5g) of solid ZnSO 4 ·7H 2 O dissolved in 120ml of H 2 In O; another 0.43mol (17.2g) of solid NaOH and 0.24mol (19.0g) of solid NH 4 HCO 3 Dissolve in 120ml of water.
[0041] Step B: Add 80ml of deionized water into a fully back-mixed liquid membrane reactor with a volume of 1L, adjust the gap of the reactor to 1 micron, start the reactor, and control the flow rate of the rotor at about 1000rpm. Add zinc salt solution and alkali solution into the reactor at the same time, and the reaction mixture stays in the reactor for 20 minutes.
[0042] Step C: Pour the synthetic reaction material flowing out of the reactor in Step B into a 500ml crystallization kettle, and crystallize at 50°C for 0.5h.
[0043] Step D: The crystallized substance is filtered, washed and dried to obtain basic zinc carbonate.
[0044] Step E: Calcining basic zinc carbonate in a high-temperature furnace at 400°C to obtain zinc oxide.
[0045] The transmission electron micro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com