Polymer chemical slow release fungicide contg. carbendazim active component
A high-molecular type, fungicide technology, applied in biocides, animal repellents, plant growth regulators, etc., can solve uneconomical problems, achieve the effects of less application times, stable chemicals, and lower agricultural costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Example 1 Synthesis of 1-hydroxyethyl-2-benzimidazole carbamate
[0024] In a 125 ml four-neck flask equipped with a stirrer, a thermometer, and a reflux condenser, add 2.0 g (0.0104 mol) of carbendazim, 1.33 g (0.0156 mol) of chloroethanol, 75 ml of absolute ethanol and an appropriate amount of Sodium ethoxide, start stirring, heat up, reflux for 1 hour and then cool to room temperature, filter, rinse the filter cake with 100 ml of 50°C distilled water, and drain to obtain 1.76 g of a light yellow solid with a yield of 72%. The crude product is purified by a silica gel column Afterwards (chloroform was the eluent) an off-white solid was obtained with a purity of 99.6%, mp.227°C (discoloration and decomposition). 1 H NMR (DMSO) δ3.86 (s, 3H, CH 3 ), 4.11(t, 2H, CH 2 ), 4.40(t, 2H, CH 2 ), 6.87-7.34 (m, 4H, aromatic), 11.71 (s, 1H, -NHCOO-); IR carbamate carbonyl characteristic absorption peak 1740cm -1 , benzene ring skeleton vibration absorption peak 1580cm -1 、15...
example 2
[0025] Example 2 Synthesis of methyl 1-ethyl (2'-acrylate-1'-yl)-2-benzimidazole carbamate
[0026] In a 125 ml four-neck flask equipped with a stirrer, a thermometer, and a reflux condenser, add 1.5 grams (0.0055 moles) of crude product 1-hydroxyethyl-2-benzimidazole carbamate, 0.56 grams (0.0056 moles) Triethylamine, dichloromethane 65 ml, start stirring, cool down to 0-5°C with an ice-water bath, add 0.5 g (0.0055 mole) of acryloyl chloride dropwise, slowly warm up to room temperature and continue stirring for 5 hours, then depressurize The solvent was distilled off to obtain a light pink solid, which was recrystallized with isopropanol to obtain 0.76 g of a light yellow solid with a purity of 98%, mp. 201°C (decomposition), and a yield of 48%. 1 H NMR (CDCl 3)δ3.62(s, 3H, CH 3 ), 4.56(t, 2H, CH 2 ), 4.50(t, 2H, CH 2 ), 6.2-64 (m, 3H, -CH=CH 2 ), 6.98-7.23 (m, 4H, aromatic), 10.08 (s, 1H, -NHCOO-).
example 3
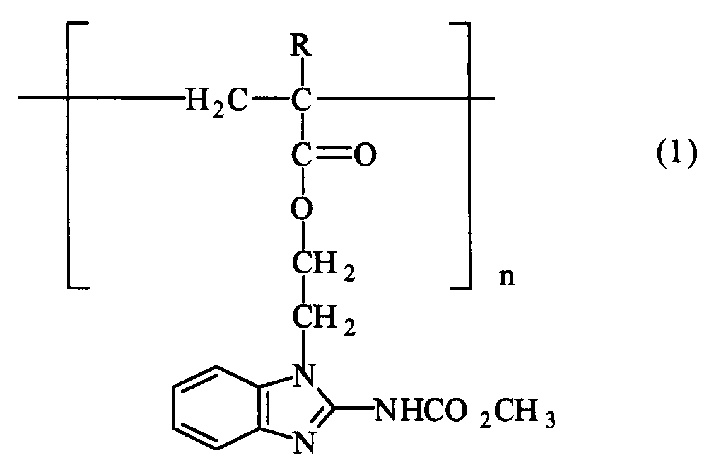
[0027] Synthesis of Example 3 Polymer Sustained Release Agent (I)
[0028] In a 250 ml three-neck flask equipped with a thermometer, stirring and reflux condenser, add 100 ml of benzene, 2.89 g of 1-ethyl(2'-acrylate-1'-yl)-2-benzimidazole carbamate 1. 0.01 g of benzoyl peroxide, slowly warming up to 60° C. under stirring, and reacting for 1 hour. Then cool down, add 80 milliliters of water, then concentrate under vacuum with stirring, reclaim benzene, filter the residue, and dry to obtain 2.3 grams of light red solid, which is polymer slow-release agent (I), with a yield of 80%. The average molecular weight is 6,500 as measured by Ubbelohde's viscometer, that is, the degree of polymerization n is 22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com