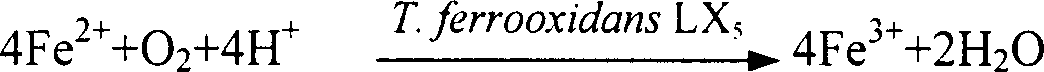Microbial-catalytic synthesizing method of pyrite ammonia alum
A microbial and jarosite technology, which is applied in the field of microbial catalyzed synthesis of jarosite, can solve the problems of low product purity, low yield, and red doping, and achieve the effects of high purity, uniform particles, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: KFe 3 (SO 4 ) 2 (OH) 6
[0019] Add 6.95g FeSO to the reactor 4 ·7H 2 O and 0.725gK 2 SO 4 , dissolved in 50ml of distilled water, adjusted to pH 2.5 with KOH or sulfuric acid; T.ferrooxidans LX 5 Bacterial cells were added to the reaction solution to make the number reach 10 7 About one / ml; under normal temperature and pressure (25°C and one atmospheric pressure) aeration and stirring for 3 days, stop stirring and let it settle naturally; collect the precipitate with ordinary filter paper, wash twice with distilled water acidified with sulfuric acid (pH1.5) , and then washed twice with distilled water until there was no SO in the washing solution 4 2- until. Dry at 50-60°C for about 3 hours to obtain the dry product KFe 3 (SO 4 ) 2 (OH) 6 3.84 g, 92% yield. The product is yellow crystal particles with uniform size, and the particle size is about 10-15μ.
[0020] The microorganism Thiobacillus ferrooxidans LX that is used for catalysis in the...
Embodiment 2
[0023] IR (KBr): cm -1 3383(OH), 1634, 1191, 1087, 627, 1004, 511, 427. This identification result is consistent with KFe 3 (SO 4 ) 2 (OH) 6 The results for the standard were in perfect agreement. Example 2: NaFe 3 (SO 4 ) 2 (OH) 6 Synthesis
[0024] Add 27.8g FeSO to the reactor 4 ·7H 2 O and 2.37gNa 2 SO 4 , dissolved in 100ml distilled water, adjusted to pH 2.0 with NaOH or sulfuric acid; T.ferrooxidans LX 5 Bacterial cells were added to the reaction solution to make the number reach about 10 8 pc / ml; under normal temperature and pressure (25°C and 1 atmospheric pressure) aeration and stirring for 2 days, stop stirring and allow it to settle naturally; the precipitate was collected with ordinary filter paper, washed twice with distilled water acidified with sulfuric acid (pH1.5), Then wash 2-3 times with distilled water until there is no SO in the washing solution. 4 2- until. Dry at 95° C. for about 2 hours to obtain 13.90 g of dried product with a yield o...
Embodiment 3
[0028] The results of this identification and NaFe 3 (SO 4 ) 2 (OH) 6 The results for the standard were in perfect agreement. Example 3: NH 4 Fe 3 (SO 4 ) 2 (OH) 6 Synthesis
[0029] Add 13.9g FeSO to the reactor 4 ·7H 2 O and 2.17g (NH 4 ) 2 SO 4 , dissolved in 100ml distilled water, adjusted to pH 2.5 with ammonia or sulfuric acid; T.ferrooxidans LX 5 Bacterial cells were added to the reaction solution to make the number reach 10 8 About one / ml; under normal temperature and pressure (25°C and one atmospheric pressure) aeration and stirring for 2 days, stop stirring and let it settle naturally; collect the precipitate with ordinary filter paper, wash twice with distilled water acidified with sulfuric acid (pH1.5) , and then washed 2-3 times with distilled water until there is no SO in the washing solution 4 2-until. Dry at 50-60° C. for about 2-3 hours to obtain 14.05 g of dry product with a yield of 88%.
[0030] The product is yellow crystal particles wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com