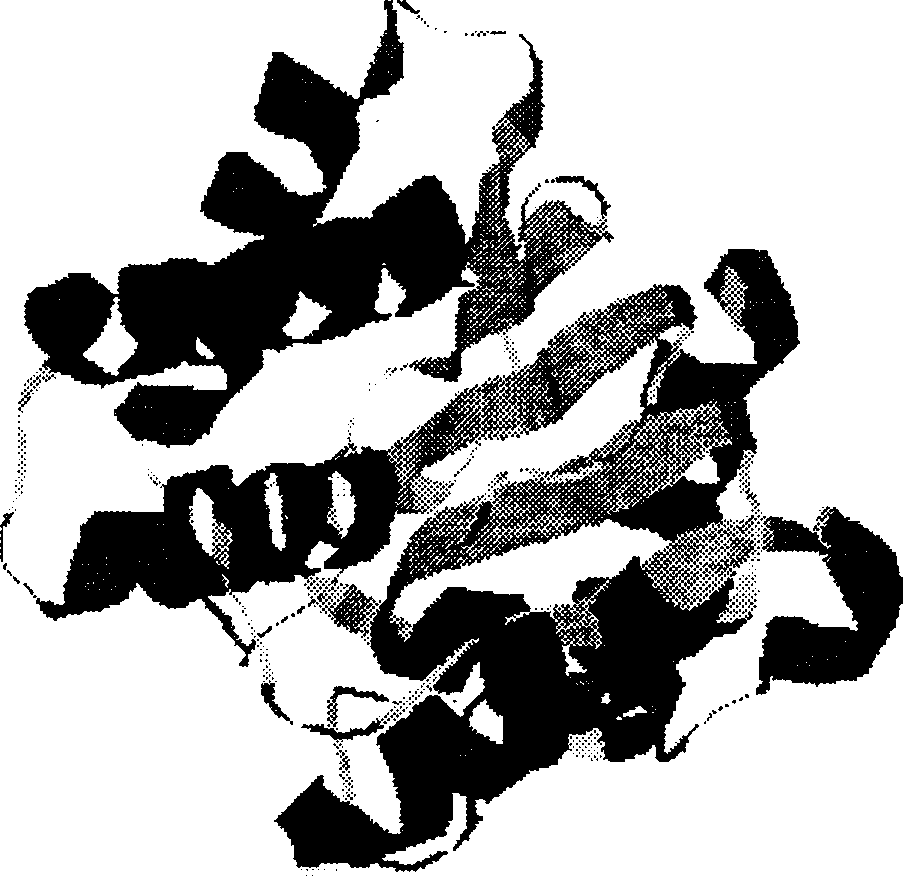
Leptospira pathogenic related protein and its code squence
A leptospira and related protein technology, applied in the fields of biotechnology and medicine, can solve the problems of undisclosed leptospira pathogenic related proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Bacterial culture
[0064] 1) Culture medium: use EMJH liquid medium to prepare 1000 ml of 5× EMJH medium, and weigh 50 g of BSA. Anhydrous sodium acetate 0.5g, sodium pyruvate 1g, ferrous sulfate 0.25g, 1.5% magnesium chloride 3.5ml, 0.4% zinc sulfate 5ml, 0.15 copper sulfate 1ml, disodium hydrogen phosphate 12.6g, potassium dihydrogen phosphate 1.5 g, sodium chloride 5g, ammonium chloride 1.25g, 0.05% VB 12 2ml, VB 1 0.025g, adjust the pH to 7.2-7.6, set the volume to 1000ml, and sterilize by filtration through nitrocellulose membranes with 0.7, 0.45, and 0.2nm pore sizes, respectively. When used, dilute it 5 times with sterile water.
[0065] 2) Culture conditions: 30° C., 100 rpm shaking culture for 40 hours.
[0066] 3) Strains: Leptospira interrogans jaundice and haemorrhagic group Lai type Lai strain.
Embodiment 2
[0068] Nucleic acid and amino acid sequences of pathogenicity-related proteins
[0069] Leptospira DNA was extracted by conventional methods to construct a library. Determining the complete sequence of the genome. According to the results of computer analysis, primers were synthesized, and the extracted Leptospira DNA was used as a template for PCR amplification to obtain the ORF sequence of the pathogenicity-related protein. Sequencing verification again, consistent with the genome sequence. These ORF codes have SEQ ID NO: 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44 , 46, 48, 50, 52, 54 amino acid sequences shown in the polypeptide. The basic information of the eight pathogenicity-related proteins of the present invention is shown in the table below.
[0070] name
ORF sequence SEQ
ID NO:
Length (bp)
protein sequence
SEQ ID NO:
length
(aa)
peptide *
collagenase ...
Embodiment 3
[0076] Protein
[0077] The Leptospira pathogenicity-related protein gene was cloned into the fusion expression vector pET28b (Novagene Company) by conventional genetic engineering method, and Escherichia coli BL21 (DE3) was selected as the recipient bacterium for fusion expression. After fermentation under conventional conditions, the recombinant bacterial protein was extracted, and then subjected to SDS-PAGE electrophoresis, and the molecular weight was basically the same as the predicted value. The pathogenicity-related protein (fusion protein with His-tag) expressed in Escherichia coli was separated and purified by Ni-NTA column to obtain pure Leptospira pathogenicity-related protein (fusion protein).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com