Medusa cystatin gene, and expression and use thereof
A technology of cysteine protease and inhibitor, applied in the field of new genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Construction of the jellyfish tentacles cDNA library
[0052] The extraction of total RNA from jellyfish tentacles refers to TRIZOL from Gibcol BRL LS reagent instructions; cDNA synthesis according to Clontech company SMART TM cDNALibrary Construction Kit manual operation.
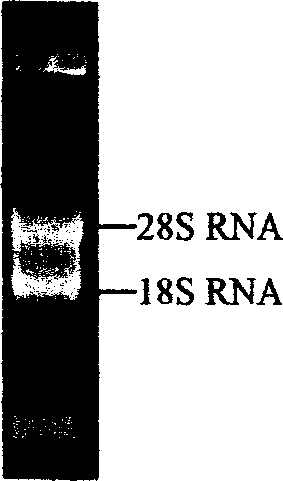
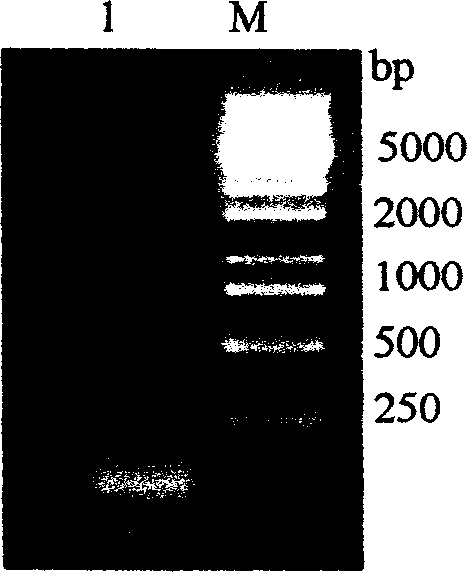
[0053] Using TRIZOL The tentacle total RNA extracted by LS reagent was detected by 1% formaldehyde denaturing gel electrophoresis as follows: figure 1 The clear 28S and 18S rRNA bands indicated that the integrity of the total RNA was good. Adopt SMART TM The cDNA synthesized by the cDNA Library Construction Kit was electrophoresed on a 1% agarose gel, and the result showed a uniform distribution, such as figure 2 As shown, the size ranged from 200bp to 5kb, mainly concentrated in the region below 2kb, indicating that the integrity of the cDNA was good. The cDNA was inserted into the plasmid vector pcDNA3 to construct a cDNA library, and the number of library clones was 5.1×10 ...
Embodiment 2
[0054] Example 2 Cloning, sequencing and analysis of jellyfish tentacles cDNA library
[0055] Select the clones of the jellyfish tentacles cDNA library, and extract the plasmid DNA according to the method of Vitagene 96-easyplasmid Mini-prep Kit. to T 7 The universal primers are sequencing primers, and the 5' ends of 2153 random cDNA sequences were sequenced using the automatic sequencer ABI Prism 3700 sequencer (Perkin-Elmer). The obtained sequences were clustered, carrier sequences removed, and Blast X analyzed. The results showed that the jellyfish tentacles cDNA library contained a variety of genes, among which the abundance of cysteine protease inhibitor genes was the highest, with 68 cDNA sequences named as Cystatin J, the jellyfish cysteine protease inhibitor gene encodes a protein of 131 amino acids, as shown in the sequence table, which includes a signal peptide of 18 amino acid residues and a mature protein of 113 amino acid residues, and the mature protein has...
Embodiment 3
[0056] Example 3 Construction of Recombinant Jellyfish Cysteine Protease Inhibitor Expression Plasmid
[0057] According to the sequence at both ends of the mature protein encoded by the Cystatin J gene and the multi-restriction site of the prokaryotic fusion expression vector pETTrx, a pair of primers were synthesized with the following sequence: upstream primer, 5'GCT GAA TTC GGT ACC CTACTT CCT GGC GGA ATA AGA CG 3' single underlined part is Kpn I restriction sequence, double underlined part is enterokinase restriction sequence downstream primer, 5'CGT GCG GCC GC AGC TCG GAG GCA TTTTGI 3' The single underline part is the Not I restriction sequence, and the double underline is the stop codon
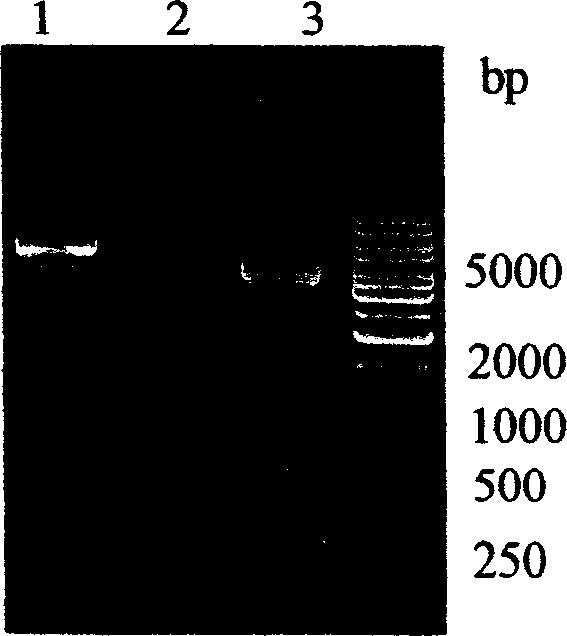
[0058] PCR amplification and gene cloning were carried out according to conventional methods. The PCR product is about 450bp such as Image 6 As shown, encoding 113 amino acid residues. The target gene was cloned into the prokaryotic fusion expression vector pETTrx to constr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com