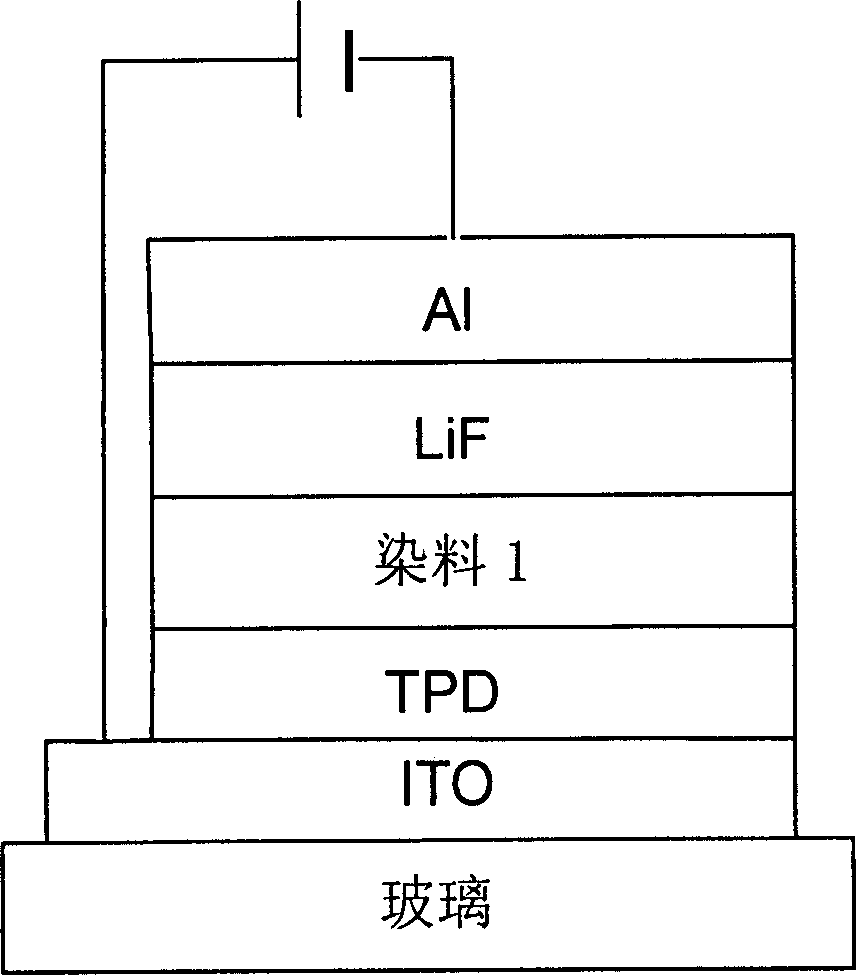
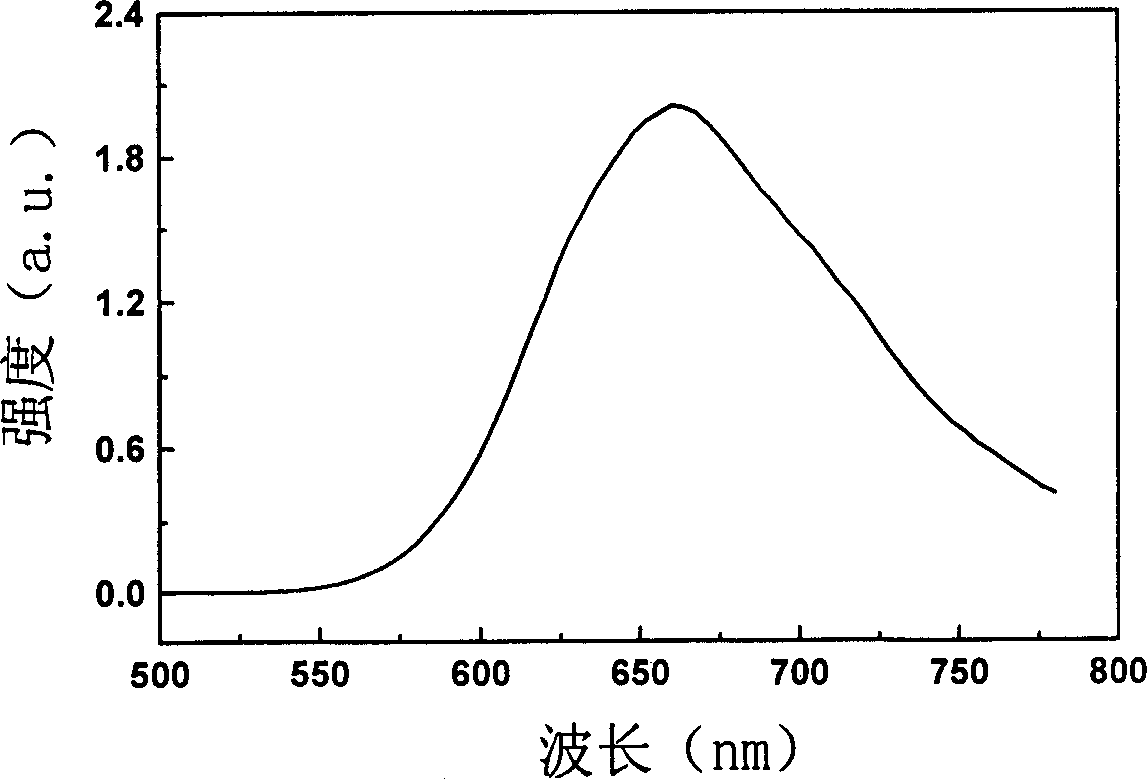
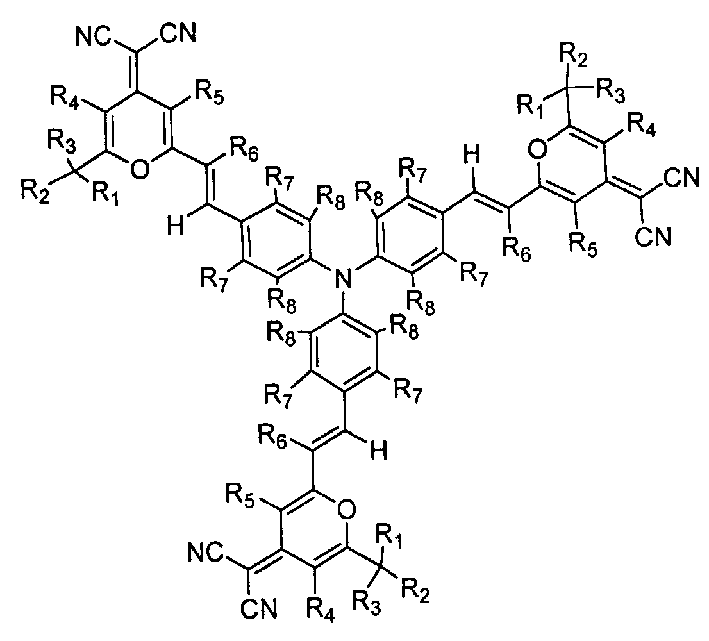
Red fluorescent dye possessing star project type structure and its synthesis method and use
A red fluorescent, star-ray technology, applied in the synthesis of fluorescent dyes, can solve the problems of not being able to effectively prevent the concentration quenching effect, complex synthesis, and increased costs, so as to reduce the concentration self-quenching phenomenon and obtain raw materials easily , the effect of long excited state lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: N, N, N-tri-4-[2-[(4-dicyanomethenyl)-8-methyl-5,6,7,8-tetrahydro-4H-1-benzo Pyran]vinyl]aniline
[0048]
[0049] In a round bottom flask, add 0.90g (4mmol) 4-dicyanomethenyl-2,8-dimethyl-5,6,7,8-tetrahydro-4H-1-benzopyran, 0.33g ( 1mmol) N,N,N-tris-(4-formyl)aniline, 15ml acetonitrile, 0.40ml hexahydropyridine, heated to reflux for 24 hours. The solvent was removed by distillation, cooled, and the solid was rinsed with acetonitrile for several times, and dried to obtain 0.67 g of the product with a yield of 70%.
[0050] NMR 1 H NMR (CDCl 3 )δ(ppm): 1.38(d, 9H, J=6.7Hz), 1.55-2.10(m, 12H), 2.80-3.10(m, 9H), 6.60(d, 3H), 6.75(s, 3H), 7.15(d, 6H), 7.30(d, 3H), 7.46(d, 6H); elemental analysis calculated value (C 63 h 51 N 7 o 3 ): C, 79.31; H, 5.39; N, 10.28;
[0051] Measured value: C, 79.18; H, 5.52; N, 10.11 mass spectrum (MS + ): 953 (M + )
Embodiment 2
[0052] Example 2: N,N,N-tris-4-[2-[(4-dicyanomethenyl)-6-isopropyl-4H-pyran]vinyl]aniline
[0053]
[0054] Add 0.70g (3.5mmol) 4-dicyanomethenyl-2-methyl-6-isopropyl-4H-pyran, 0.33g (1mmol) N,N,N-tri-( 4-formyl)aniline, 15ml of acetonitrile, 0.40ml of catalyst (the preparation method is: 5ml of hexahydropyridine dissolved in 15ml of acetic acid), heated to reflux for 12 hours. The solvent was distilled off, cooled, and the solid was rinsed with acetonitrile for several times and dried to obtain 0.59 g of the product with a yield of 65%.
[0055] NMR 1 H NMR (CDCl 3 )δ (ppm): 1.42 (d, 18H), 2.8 (m, 3H), 6.59 (d, 3H), 6.72 (s, 3H), 6.81 (s, 3H), 7.15 (d, 6H), 7.32 ( d,3H), 7.51(d,6H)
[0056] Elemental analysis calculated value (C 57 h 45 N 7 o 2 ): C, 78.15; H, 5.18; N, 11.19
[0057] Found: C, 78.32; H, 5.05; N, 11.41
[0058] Mass spectrometry (MS + ): 875 (M + )
Embodiment 3
[0059] Example 3: N,N,N-tris-4-[2-[(4-dicyanomethenyl)-6-isopropyl-4H-pyran]vinyl]aniline
[0060]
[0061] Add 0.70g (3.5mmol) 4-dicyanomethenyl-2-methyl-6-isopropyl-4H-pyran, 0.33g (1mmol) N,N,N-tri-( 4-formyl) aniline, 15ml acetonitrile, 0.40ml hexahydropyridine, heated to reflux for 12 hours. The solvent was removed by distillation, cooled, and the solid was rinsed with acetonitrile for several times, and dried to obtain 0.66 g of the product with a yield of 75%.
[0062] NMR 1 H NMR (CDCl 3 )δ (ppm): 1.40 (d, 18H), 2.8 (m, 3H), 6.60 (d, 3H), 6.72 (s, 3H), 6.81 (s, 3H), 7.15 (d, 6H), 7.32 ( d,3H), 7.51(d,6H)
[0063] Elemental analysis calculated value (C 57 h 45 N 7 o 2 ): C, 78.15; H, 5.18; N, 11.19
[0064] Found: C, 78.21; H, 5.15; N, 11.38
[0065] Mass spectrometry (MS + ): 875 (M + )
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com