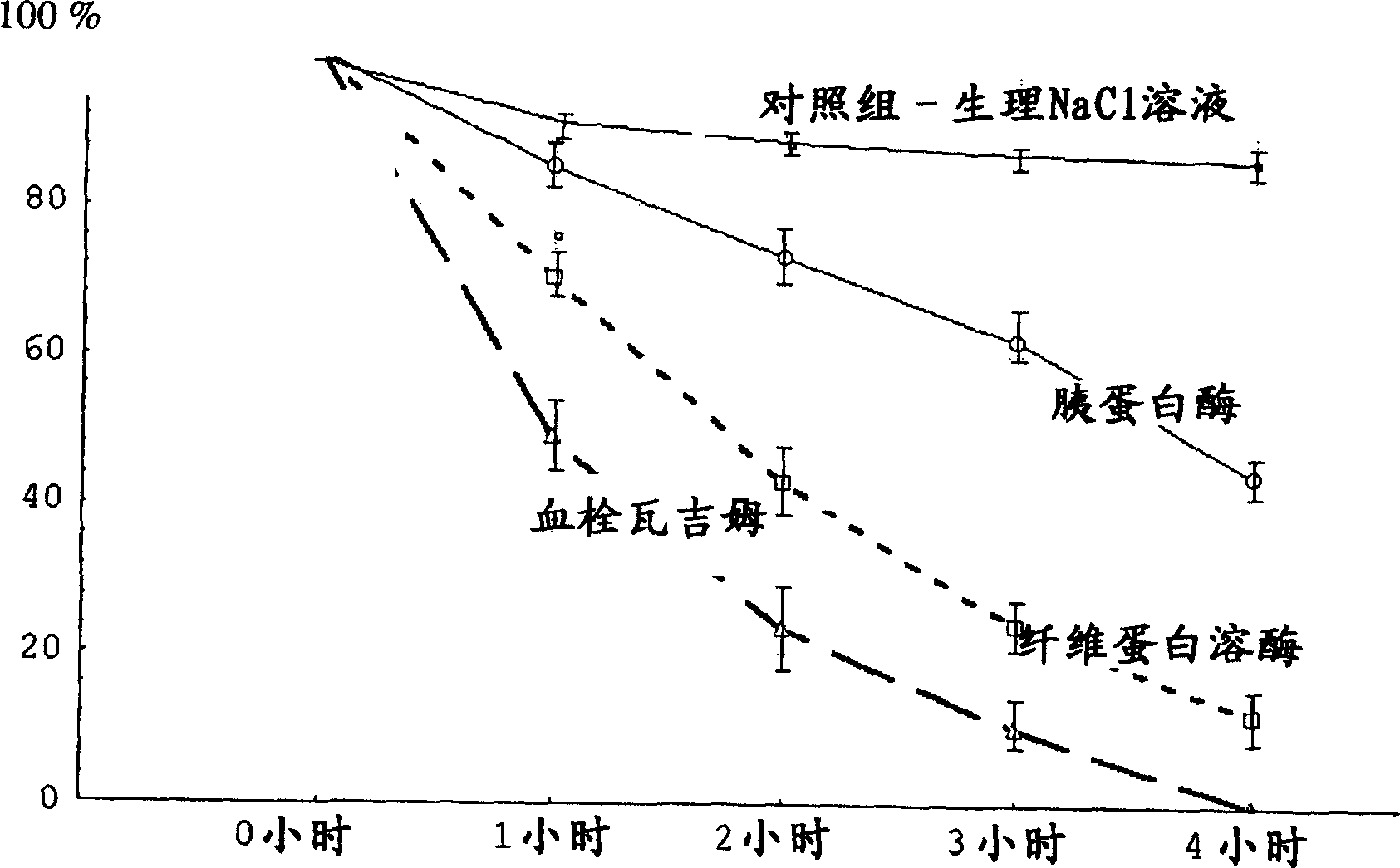
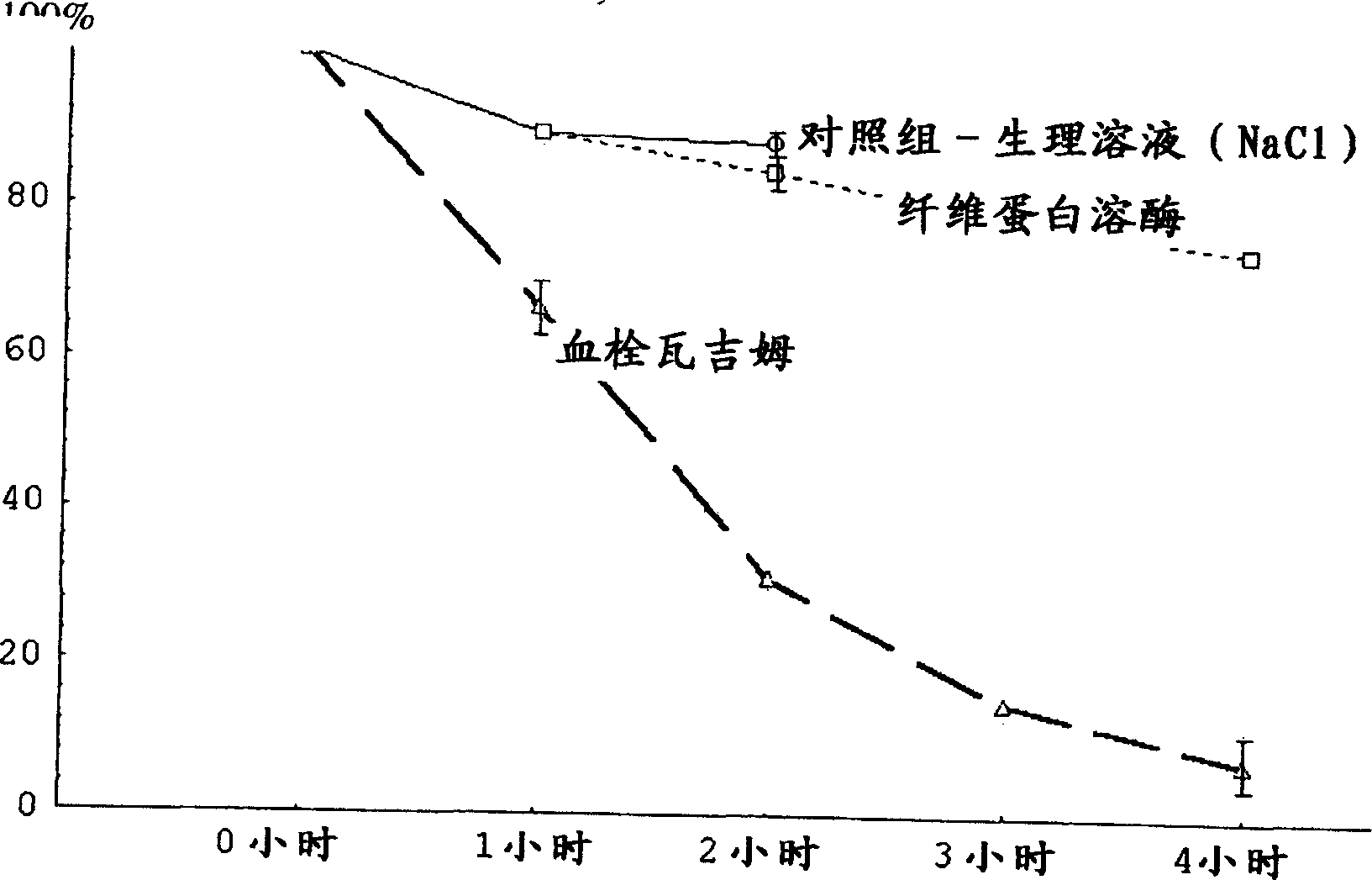
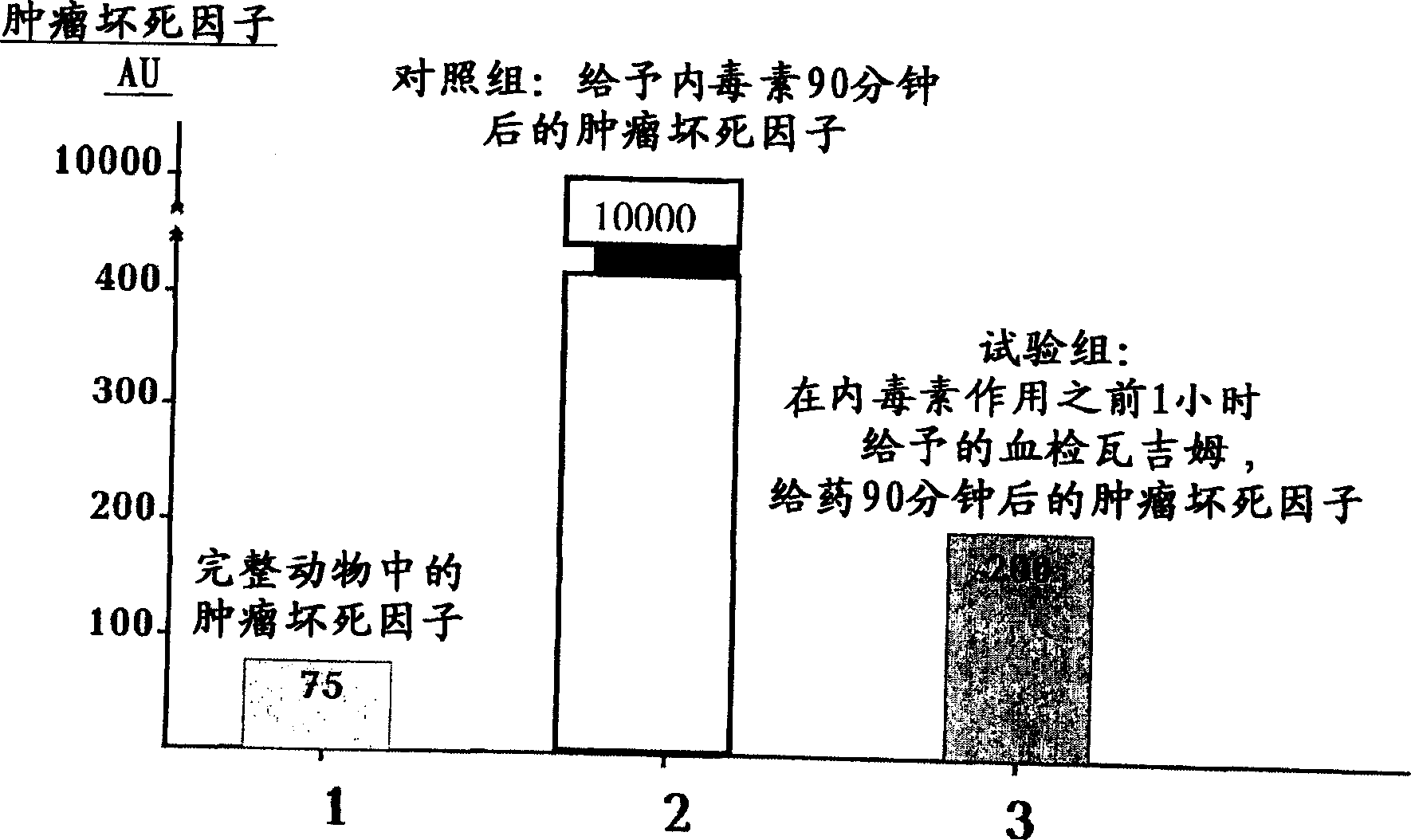
Drug compastion with thrombolysis, anti-inflammatory and cell protective function
A technology of water-soluble polymers and compositions, applied in the field of blood fibrin and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Prepare the pharmaceutical composition of the present invention by the following method
[0039] The method for preparing the reaction mixture is to dissolve protosubtilin (protosubtilin) in 0.025M sodium phosphate buffer solution (its pH value is 7.5-8.2) in a dextran (molecular weight is 40-70kDa, preferably 40kDa) solution, Preference is given to prosubtilisin G3Kh and polyethylene oxide (PEO) (with a molecular mass of 400-20000 Da, preferably 1500 Da). The resulting mixture was purified by removal of inert and useless proteins by salt precipitation followed by filtration. The resulting solution was irradiated with γ-rays or accelerated electron beams (energy 2.0 MeV) at a dose of 0.5-1.5 Mrad. After irradiation the solution was sterile filtered and dispensed in 10 ml portions into vials with a capacity of 15 ml each. Then the solution was dried by lyophilization until the residual moisture was not more than 2%.
[0040] The result was a composition containing a...
Embodiment 2
[0043] The composition of the present invention can be prepared by another method, that is, to prepare respectively the active component (the first component) containing the protease complex immobilized on polyoxyethylene and dextran and the solvent—polyoxyethylene solution ( Component 2). By changing the ratio of active component / solvent, the two components of the composition can change the activity of the pharmaceutical composition of the present invention, so that various individual treatment schemes can be selected.
[0044] Adopt following way to prepare above-mentioned two-component composition:
[0045] Component 1 (active substance)
[0046] The reaction mixture was prepared by dissolving prosubtilin (preferably prosubtilin) in 0.025 M sodium phosphate buffer solution (pH 7.5-8.2) of dextran (molecular weight 40-70 kDa, preferably 40 kDa) G3Kh) and polyethylene oxide (PEO) with a molecular weight of 400-20000 Da (preferably 1500 Da). The resulting mixture is purifi...
Embodiment 3
[0054] 15g polyoxyethylene PE0-1500 is dissolved in the 0.025M phosphate buffer (pH is 7.5) solution of the dextran of 300ml 10%, the molecular weight of dextran is 40kDa, adds 6.3g prosubtilin G3Kh, at 18 -20°C, the mixture was stirred for 30 minutes. Salt precipitation is then used to precipitate inert and useless proteins. To this end, 1.3 g of Sodium phosphate disubstituted were sequentially added to the mixture to dissolve completely and to a final concentration of 0.45% and 1.9 g of calcium chloride to a final concentration of 0.63%. After calcium chloride is dissolved in the reaction mixture, an insoluble calcium phosphate precipitate settles out, which adsorbs inert and useless proteins. The mixture was kept at 4-8°C for 12 hours to completely precipitate inert and useless proteins. The reaction mixture was then filtered through filter paper (white band). The filtrate volume was 300ml. The resulting solution was irradiated with gamma rays at a dose of 1.0 Mard. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com