Slow and control release aspirin capsule formulation and method for making same
A technology of aspirin and capsules, which is applied in the field of aspirin sustained and controlled release capsule preparations and its preparation, can solve the problems of large impact, aggravated side effects, unstable blood drug concentration, etc., and achieve the effect of improving curative effect and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
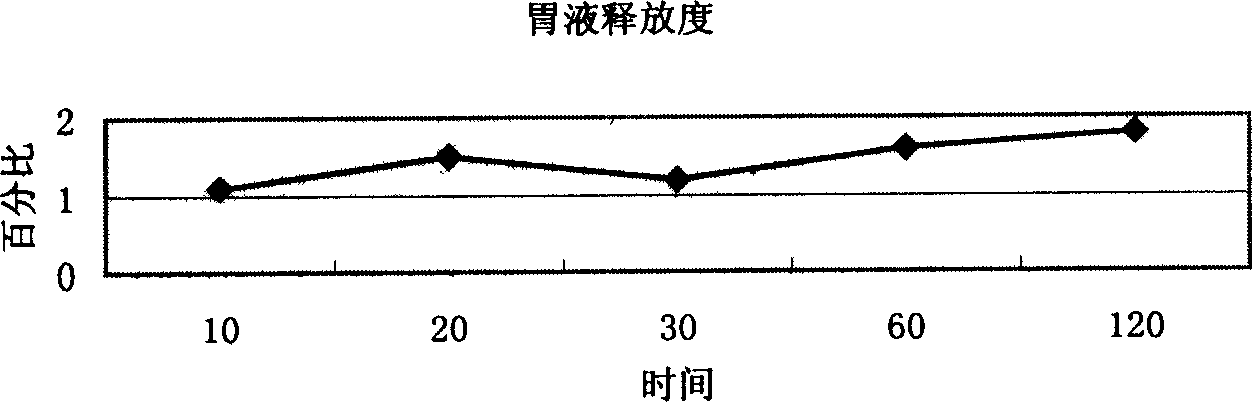
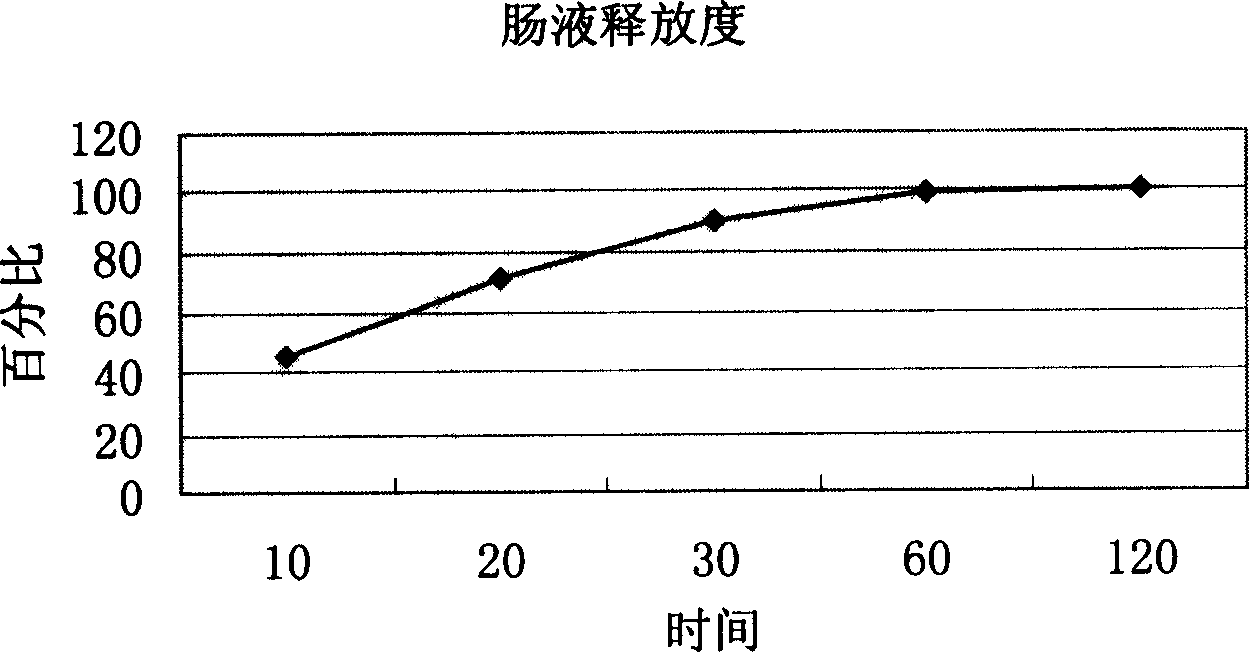
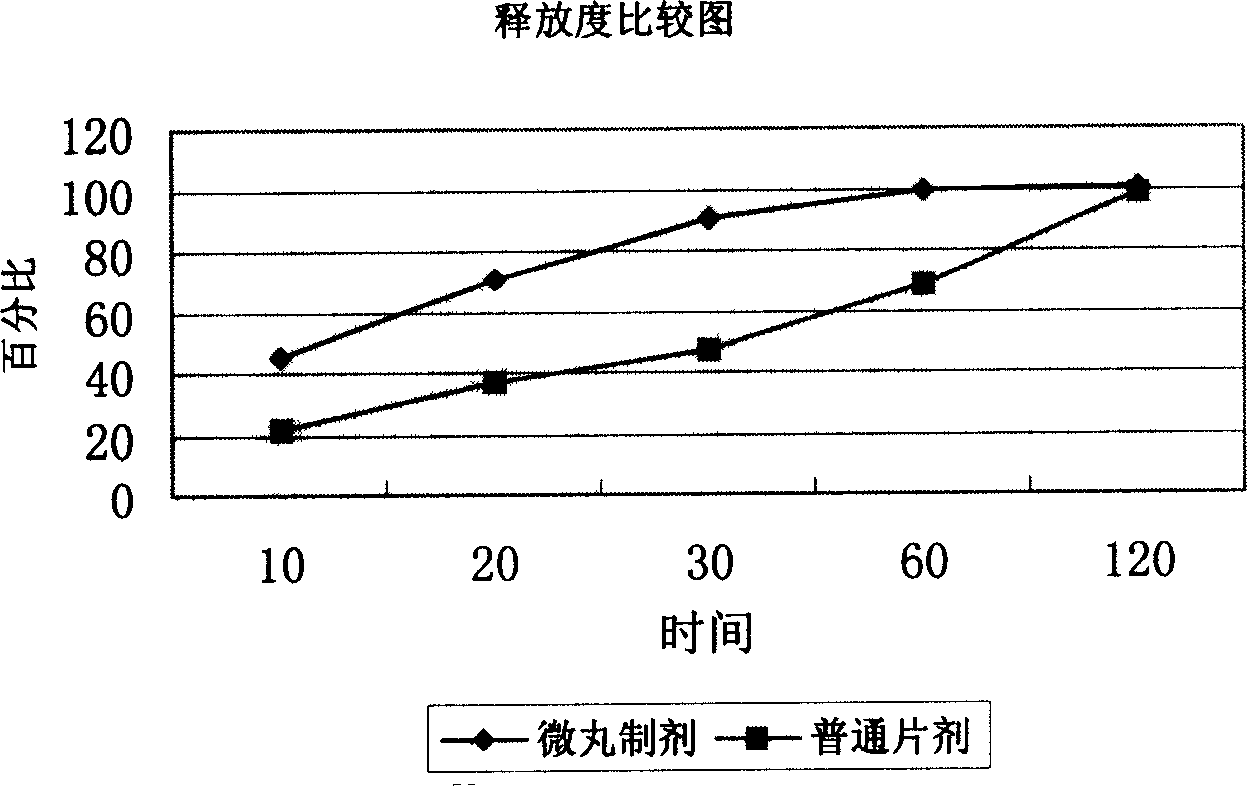
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of ball core
[0036] The ball core used in the present invention can adopt commercially available blank ball core, also can adopt telecentric type coating machine, fluidized bed coating machine or the water chestnut type sugar-coated pan after transformation to prepare by oneself by following prescription. The preferred diameter is 450μm-780μm, and the bulk density is 0.75-0.95g / ml.
[0037] Homemade blank ball core prescription:
[0038] Sucrose (or lactose) 1-4Kg
[0039] Starch (or microcrystalline cellulose) 1-4Kg
[0040] 30-66% sucrose for sucrose syrup 3-5Kg
[0041] (or 1-4% hypromellose, 3-10% povidone or 3-10% polyethylene glycol) 0.1-0.5Kg
[0042] Preparation:
[0043] Grind sucrose (or lactose) through a 150 μm sieve, starch (or microcrystalline cellulose) through a 150 μm sieve, and prepare a mixed powder according to the prescription ratio. The sucrose syrup is heated and dissolved with sucrose plus the prescribed amou...
Embodiment 2
[0044] Embodiment 2: Preparation and coating of aspirin-containing pellets
[0045] The aspirin-containing pellets of the present invention can be prepared according to the following prescription using a telecentric coating machine or a modified sugar coating pan.
[0046] Prescription containing aspirin pellets:
[0047] Blank ball core 13-18Kg
[0048] Aspirin 6-10Kg
[0049] Starch (or microcrystalline cellulose) 1-4Kg
[0050] Sucrose (or lactose) 1-4Kg
[0051] 30-66% sucrose syrup 5-7Kg
[0052] (or 1-3% hypromellose, 3-10% povidone or 3-10% polyethylene glycol) 0.1-0.5Kg
[0053]Grind aspirin to a size of 150 μm, starch (microcrystalline cellulose) to a size of 150 μm, and sucrose (lactose) to a size of 150 μm, and make a mixed powder according to the prescription ratio. Heat and dissolve sucrose syrup with sucrose plus prescribed amount of purified water (or prepare hydroxypropylmethylcellulose, povidone, polyethylene glycol according to the concentration) and pa...
Embodiment 3
[0061] Embodiment 3: Preparation and coating of blank pellets
[0062] The blank pellets in the present invention can be prepared by using a telecentric coating machine or a modified sugar pan according to the following prescription.
[0063] Blank ball core 10-15Kg
[0064] Starch (or microcrystalline cellulose) 4-8Kg
[0065] Sucrose (or lactose) 4-8Kg
[0066] 30-66% sucrose for sucrose syrup 4-6Kg
[0067] (or 1-3% hypromellose, 3-10% povidone or 3-10% polyethylene glycol) 0.25-1.25Kg
[0068] The starch (microcrystalline cellulose) is crushed through 150 μm, the sucrose (lactose) is crushed through 150 μm, and mixed powder is made according to the prescription ratio. Heat and dissolve sucrose syrup with sucrose plus prescribed amount of purified water (or prepare hydroxypropylmethylcellulose, povidone, polyethylene glycol according to the concentration) and pass through a 150 μm sieve. Put the blank ball cores into the telecentric coating machine or the modified suga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com