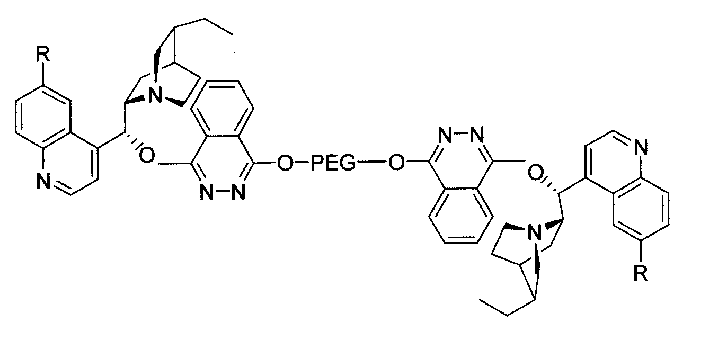
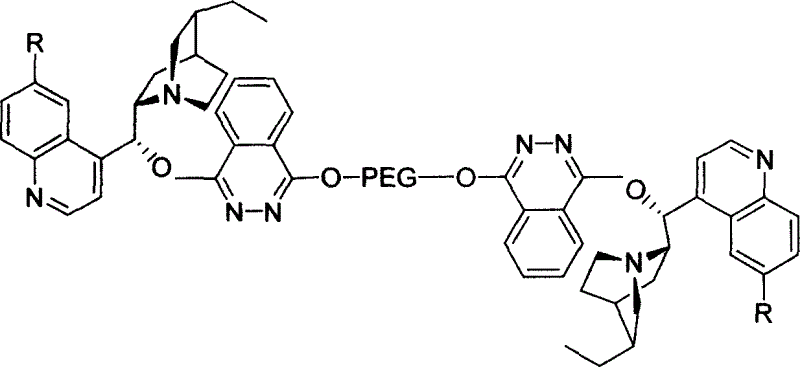
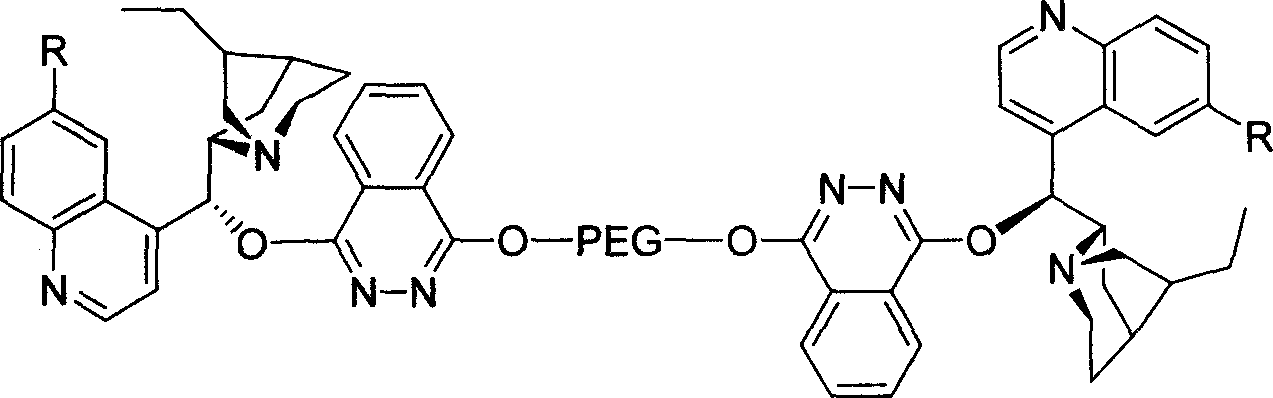
Macromolecule immobilized cinchonine alkaloid ligand, synthesis method and use thereof
A synthetic method and polymer technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve high import costs, low enantioselectivity, and long reactions Time and other issues, to achieve the effect of not reducing activity and selectivity, high yield and selectivity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of 2:
[0032] Method a: Add o-phthalic anhydride (37 grams, 0.25 mol) into a 1-liter reaction flask, add ethanol to partially dissolve the solid, then slowly add 85% hydrazine hydrate (13-15 g) dropwise, after the dropwise addition, react The system was refluxed for 4-7 hours, cooled to room temperature, filtered, and the obtained solid was washed with hot ethanol for 3-5 times, and then washed with diethyl ether for 3-4 times to obtain a white solid, which was dried and the yield was 95%.
[0033] Method b. Add phthalic anhydride (91 grams), hydrazine sulfate (80 grams), sodium acetate (10 grams), 10% glacial acetic acid (1800 milliliters) in 2 liters of reaction flasks, this mixture is stirred Heat to 80-100°C, reflux for 5-7 hours, and let stand to cool. The reaction mixture was filtered, washed with 500-1000 ml of 1% HOAc, then washed with 500-1000 ml of distilled water, 500 ml of 95% ethanol, 500 ml of anhydrous acetic acid, and 500 ml of anhydrous ethe...
Embodiment 2
[0037] Synthesis of 3:
[0038] In a 1 liter dry reaction flask, add PCl under nitrogen protection 5 (218 grams), 2 (81.0) grams, then slowly raise the temperature to 120-140 ° C, keep this temperature constant, all the solids will melt within 3-4 hours, stir for another 3 hours, and then evaporate the liquid under reduced pressure to obtain a solid the remains. The residue was dissolved by adding about 500-700 ml of dichloromethane, filtered to remove insoluble matter, added 50-100 g of basic alumina to the filtrate, stirred for 1-3 h, then filtered and dried to obtain a crude product, which was weighed in tetrahydrofuran Crystallized to obtain 1,4-dichloroazyridine with a yield of 60%.
[0039] EI-MS (m / z, %) M+198;
[0040] 1 H (300Hz, CDCl3 ): δ8.10(d, 2H), 8.45(d, 2H).
Embodiment 3
[0042] Synthesis:
[0043] Weigh 10g of quinine, add it to a 500ml flask, add about 250ml of absolute ethanol, 1.5-2.0g of 10% Pd / C, hydrogenate at normal pressure for 48h, filter to remove Pd / C, and concentrate the filtrate to obtain hydrogenated quinine . Yield 100%.
[0044] EI-MS (m / z, %) M+326;
[0045] 1 H (300Hz, CDCl 3 ): δ8.5(d, 1H), 8.0(d, 1H), 7.5(d, 1H), 7.0-7.2(m, 2H), 5.5(d, 1H), 3.9(s, 3H), 2.2- 2.4 (m, 4H), 1.2-1.9 (m, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com