Binuclear macrocyclic polyamine metal complex and its use
A technology of metal complexes and macrocyclic polyamines, applied in the field of imitation enzyme catalysts, can solve problems such as poor catalytic hydrolysis activity, and achieve the effects of easy raw materials, simple preparation methods, and expanded scope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
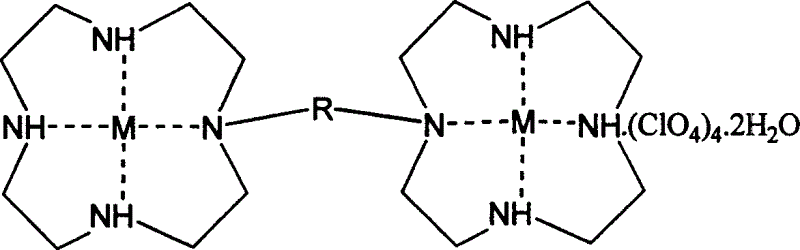
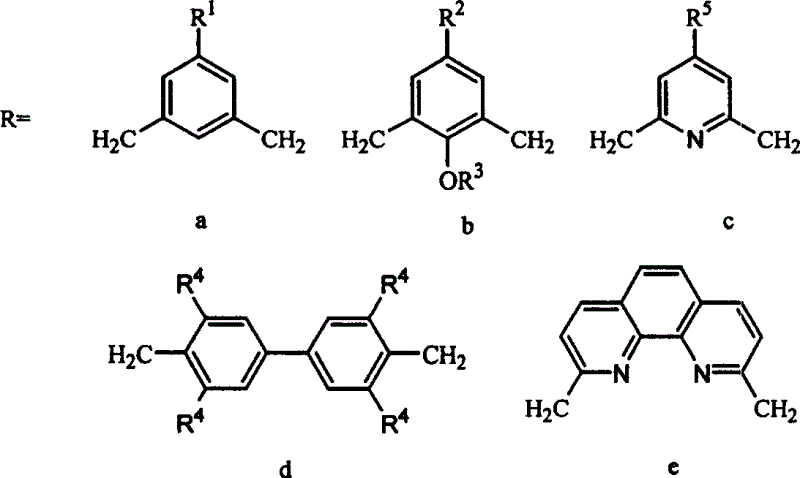
[0031] This embodiment prepares the dinuclear macrocyclic polyamine Zn (II) complex, the bridge R is a group c, and the structural formula is as follows:
[0032]
[0033] R 5 =H
[0034] The process steps are:
[0035] 1. Synthesis of macrocyclic polyamines - synthesis of 4,7,10-tris(tert-butoxycarbonyl)-1,4,7,10-tetraazadodecane
[0036] N 2 Under air protection, 146.5 grams (0.26mol) of compound-N,N',N"-tris(p-toluenesulfonyl)-diethylenetriamine was dissolved in 300ml of anhydrous DMF, and stirred in an ice bath, Add 17.1 grams (0.57mol) of sodium hydride (80%-mineral oil) in batches, and after stirring for 2 hours, add 147.0 grams (0.26mol) of compound-O, O', N-three (p-toluenesulfonyl)-bis(2-hydroxyethyl)amine in anhydrous DMF solution 300ml, stirred for 3 hours, placed at room temperature, the mixture was poured into 1000ml of ice water, and the solid was collected by filtration.Water, ethanol washing, The white solid obtained was 1,4,7,10-te...
Embodiment 2
[0045] This embodiment prepares the dinuclear macrocyclic polyamine Zn (II) complex, the bridge R is a group b, and the structural formula is as follows:
[0046]
[0047] R 2 =CH 3 R 3 =H
[0048] The process steps are:
[0049] 1. Synthesis of macrocyclic polyamines
[0050] Same as Example 1.
[0051] 2. Synthesis of bridged macrocyclic polyamine bromate
[0052] N 2 Under air protection, 1.0 g (2.12 mmol) of compound-4,7,10-tris(tert-butoxycarbonyl)-1,4,7,10-tetraazacyclododecane was dissolved in 50 ml of anhydrous acetonitrile , add 1.06 mmol 2,6-dichloromethyl-4-methylphenol and 0.25 g (2.36 mmol) anhydrous Na 2 CO 3 , reflux for 72h, filter to remove insoluble matter, distill off the solvent, dissolve the residue in water, CHCl 3 Extraction, combined organic phase, with anhydrous Na 2 SO 4 Dry and remove solvent. The residue was dissolved in anhydrous and alcohol-free chloroform, and under ice-cooling, 0.3 g (0.21 mmol) of triethylam...
Embodiment 3
[0057] This embodiment prepares the dinuclear macrocyclic polyamine Zn (II) complex, the bridge R is a group b, and the structural formula is as follows:
[0058]
[0059] R 2 = Cl R 3 =H
[0060] The process steps are:
[0061] 1. Synthesis of macrocyclic polyamines
[0062] Same as Example 1.
[0063] 2. Synthesis of bridged macrocyclic polyamine bromate
[0064] N 2 Under air protection, 1.0 g (2.12 mmol) of compound-4,7,10-tris(tert-butoxycarbonyl)-1,4,7,10-tetraazacyclododecane was dissolved in 50 ml of anhydrous acetonitrile , add 1.06 mmol 2,6-dichloromethyl-4-chlorophenol and 0.25 g (2.36 mmol) anhydrous Na 2 CO 3 , reflux for 72h, filter to remove insoluble matter, distill off the solvent, dissolve the residue in water, CHCl 3 Extraction, combined organic phase, with anhydrous Na 2 SO 4 Dry and remove solvent. The residue was dissolved in anhydrous and alcohol-free chloroform, and under ice-cooling, 0.3 g (0.21 mmol) of triethyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com