Slow-releasing microball with nimoldipine and its preparing method
A nimodipine-loaded, slow-release microsphere technology, applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., to achieve the effects of reducing clogging, low production costs, and stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] 2g of sodium alginate and 0.5g of konjac glucomannan were dissolved in 100ml of deionized water, and 2.2g of nimodipine solid dispersion was added. The weight ratio of nimodipine to 6,000 polyethylene glycol in the solid dispersion was 1: 1. Prepare a konjac glucomannan-sodium alginate mixed solution containing nimodipine solid dispersion. Using capillary crushing method, vibration frequency is 50Hz, flow rate is 138cm / s, the above mixed solution is dropped into CaCl in the form of tiny droplets 2 The concentration is 0.2M, the concentration of chitosan is 0.25% (w / v), and the molecular weight of chitosan is 8.55×10 6 In the chitosan calcium chloride solution, gel for 30 minutes, filter and wash, and dry at 50°C to obtain nimodipine sustained-release microspheres with higher strength and good controlled release properties ( figure 1 ). The average particle size of the microspheres is 0.91mm, the particle size deviation is 10.7%, and the sphericity is good.
example 2
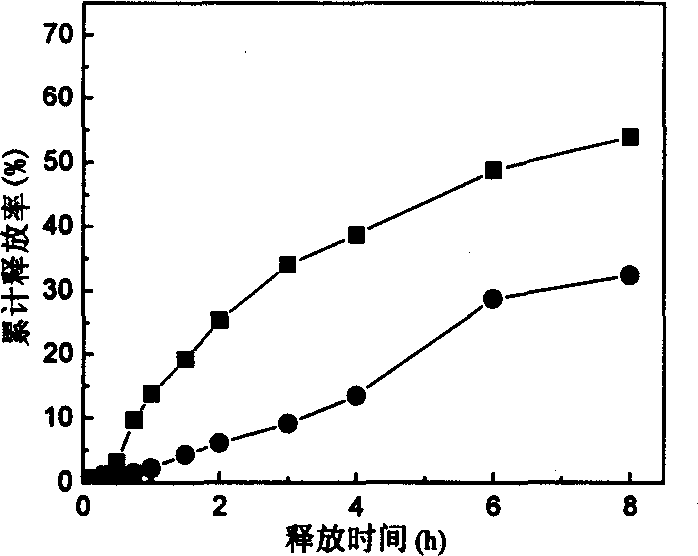
[0021] 2g of sodium alginate and 0.5g of konjac glucomannan were dissolved in 100ml of deionized water, and 2.2g of nimodipine solid dispersion was added. The weight ratio of nimodipine to 6,000 polyethylene glycol in the solid dispersion was 1: 1. Or add 1.1 g of nimodipine plain medicine to the above mixed sol to prepare a konjac glucomannan-sodium alginate mixed solution containing nimodipine solid dispersion or nimodipine plain medicine. According to Example 1, the capillary crushing method was used to prepare sustained-release microspheres containing solid dispersion of nimodipine and nimodipine. After treatment in simulated gastric juice for 4 hours, the gastric juice release rate of the microspheres containing nimodipine and nimodipine solid dispersion were 3.75% and 4.56%, respectively. The microspheres are placed in the simulated intestinal fluid again, and the release of the drug can be seen figure 2 . figure 2 It can be seen that the release rate of the microspheres l...
example 3
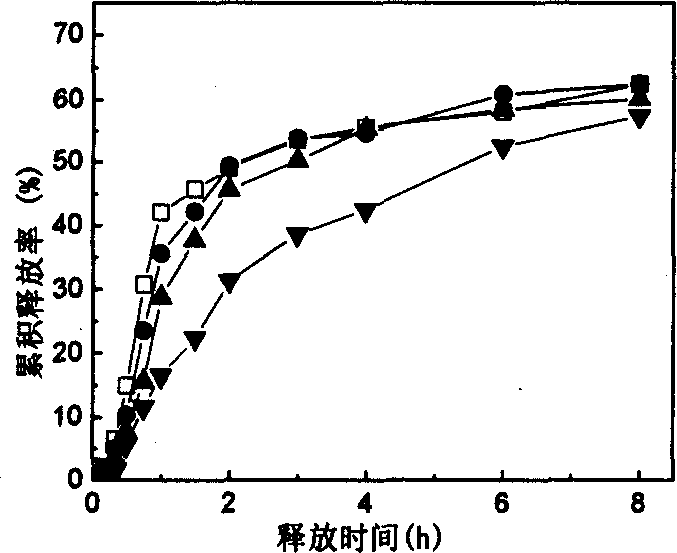
[0023] 2g of sodium alginate and 0, 0.25, 0.375, 0.5g of konjac glucomannan were dissolved in 100ml of deionized water, and 2.2g of nimodipine solid dispersion was added respectively. The solid dispersion of nimodipine and the molecular weight of 6000 The weight ratio of polyethylene glycol is 1:1, and nimodipine solid dispersion-konjac glucomannan-sodium alginate mixed solutions with different contents of konjac glucomannan are prepared. According to Example 1, the capillary crushing method was used to prepare drug-loaded konjac glucomannan-calcium alginate-chitosan microspheres with different contents of konjac glucomannan. After 4 hours of treatment in simulated gastric juice, the gastric juice release rate of the above microspheres was all lower than 5%. The particles are again placed in the simulated intestinal fluid, and the release of the drug can be seen image 3 . by image 3 It can be seen that the microspheres containing a higher concentration of konjac glucomannan have...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com