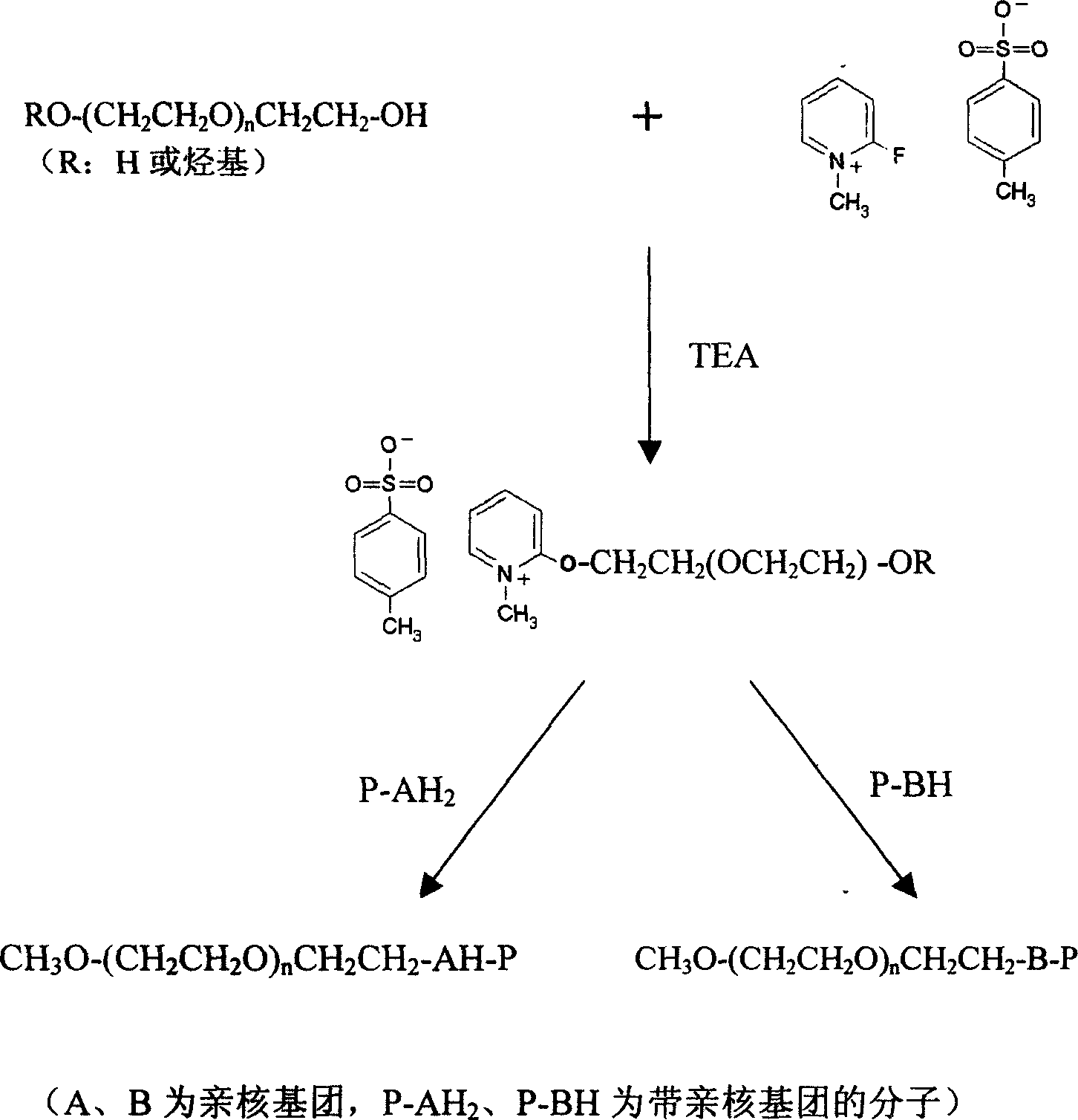
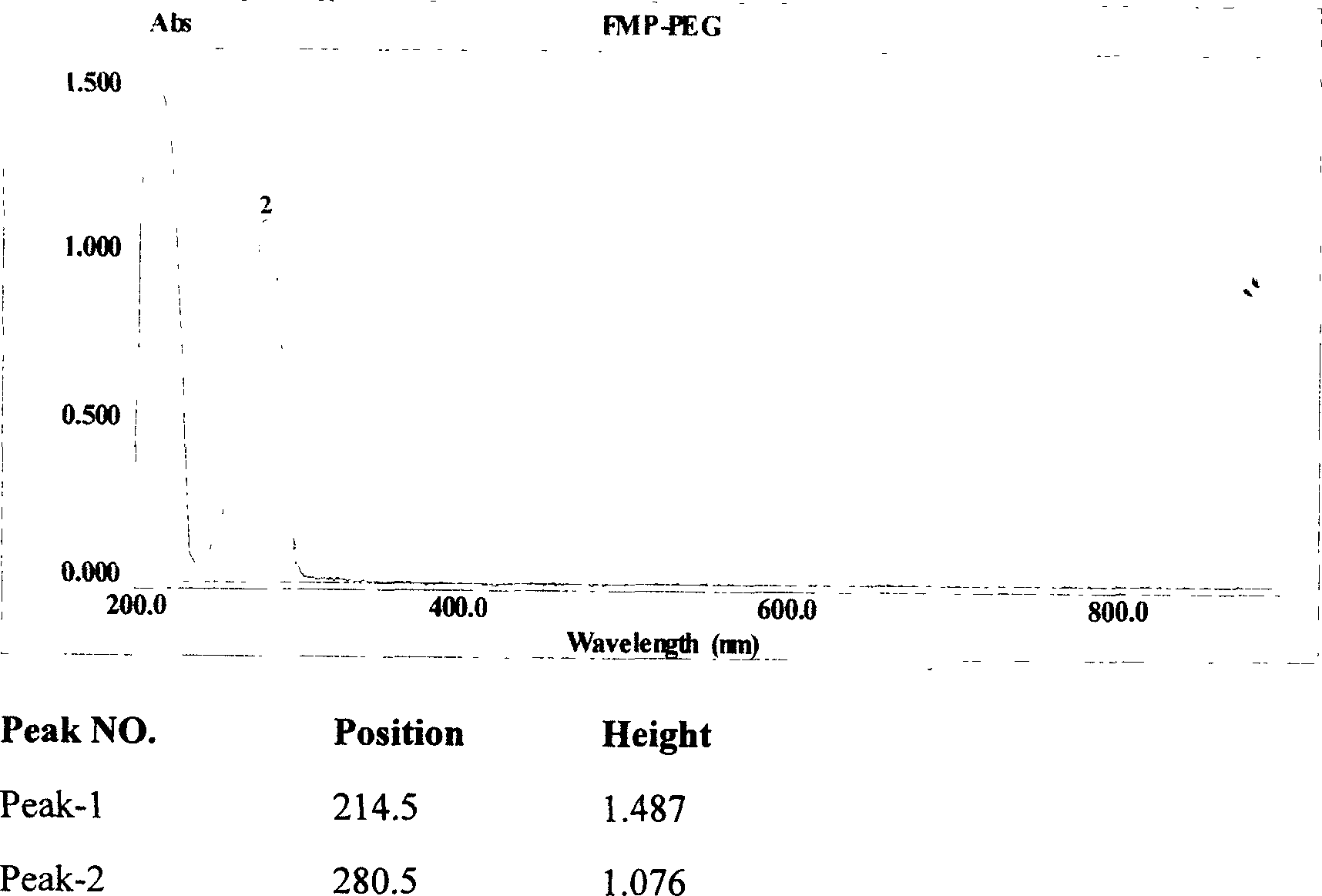
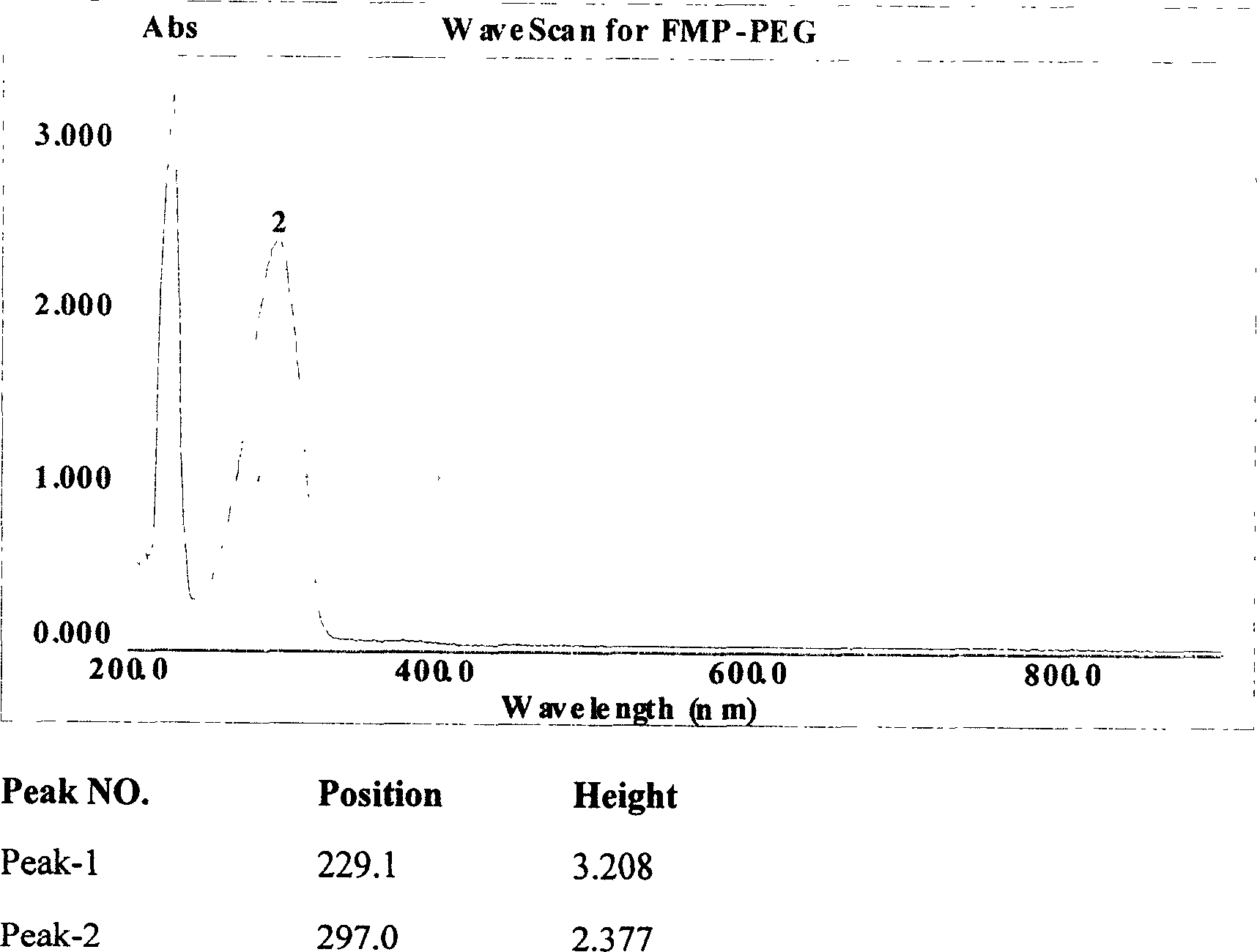
Efficent polyethylene glycol activating process and activate use for protein modification
A polyethylene glycol and activated product technology, applied in the field of protein chemistry, can solve the problems of long half-life and low clearance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Activation of monomethoxypolyethylene glycol 5000 (mPEG5000) with 2-fluoro-1-methylpyridinium toluene-4-sulfonate (FMP)
[0064] Add 250ml of toluene and 50g of mPEG5000 into a 1000ml round bottom flask, connect the water separator, reflux condenser and drying tower to the round bottom flask in turn, and then heat the toluene solution to 140°C. Keep at reflux for two hours. Then change the reflux device to a vacuum distillation device, and steam 200ml of toluene. Add 500ml of diethyl ether to the round-bottomed flask again, and cool down naturally under the condition of stirring until the solid completely precipitates out. The ether was then removed by filtration, and the precipitate was placed in a vacuum desiccator to remove residual ether. Dry anhydrous mPEG5000 was obtained. Then measure the moisture content in the dried anhydrous mPEG5000 with a Karl Fischer moisture analyzer, and the moisture content is required to be lower than 50ppm.
[0065] Weigh and dry 2...
Embodiment 2
[0068] Conjugate recombinant human granulocyte colony-stimulating factor (rhG-CSF) with FMP-mPEG5000
[0069] G-CSF was dissolved in 0.1M boric acid-borax buffer solution, pH value was 8.6, and the concentration was 2.3 mg / ml. Take 40ml of the solution and pour it into a 200ml Erlenmeyer flask and shake it in a constant temperature shaker at 25°C. Then 0.98 g of FMP-mPEG5000 was added to the Erlenmeyer flask. After the reaction lasted for 30 minutes, the pH value of the reaction system was adjusted to 4.0 to terminate the coupling reaction. Then use gel filtration chromatography to remove unreacted FMP-mPEG, and the coupled product collected is analyzed by SDS-PAGE for its molecular weight change, the results are as follows Figure 4 As shown, the molecular weight of the product modified with FMP-mPEG increased, indicating that a coupling reaction occurred between FMP-mPEG and G-CSF, and a coupled product was obtained.
Embodiment 3
[0071] Conjugate recombinant human α-interferon (rhα-Interferon) with FMP-mPEG5000
[0072] Dissolve α-interferon in 0.1M boric acid-borax buffer solution, the pH value is 7.6, and the concentration is 3.5 mg / ml. Take 40ml of the solution and pour it into a 200ml Erlenmeyer flask and place it in a constant temperature shaker at 25°C for gentle shaking. Then 3.1 g of FMP-mPEG5000 was added to the Erlenmeyer flask. After the reaction lasted for 30 minutes, the pH value of the reaction system was adjusted to 4.0 to terminate the coupling reaction. Then gel filtration chromatography was used to remove unreacted FMP-mPEG, and the collected coupled products were analyzed by SDS-PAGE for molecular weight changes. The result is as Figure 4 As shown, the molecular weight of the product modified with FMP-mPEG increases, indicating that a coupling reaction between FMP-mPEG and α-interferon occurred, and a coupled product was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com