Synthesis of benzoxazole
A technology of benzoxazole diamine and synthesis method, which is applied in the field of synthesis technology of benzoxazole diamine, and can solve the problems of easy oxidation and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
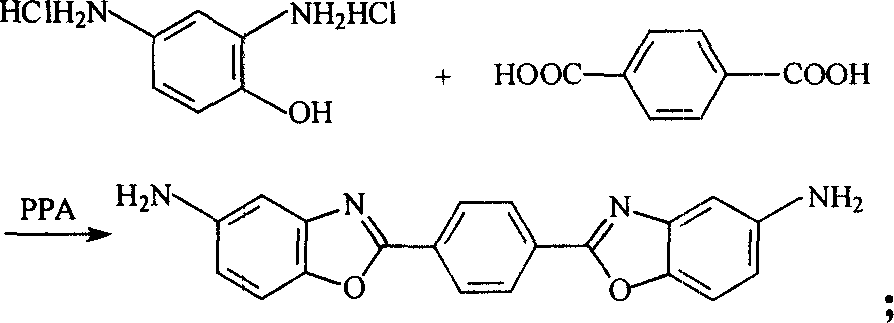
[0009] Specific embodiment one: p-phenylene-2 of the present embodiment, 2'-bis (5-aminobenzoxazole) is prepared like this: a, under inert gas protection atmosphere, phosphoric acid and P 2 o 5 Mix and configure polyphosphoric acid solution, wherein P in the solution 2 o 5 The concentration is controlled between 80% and 85%; b. Control the temperature at 60-120°C, add 2,4-diaminophenol hydrochloride and terephthalic acid reaction raw materials to the polyphosphoric acid solution, wherein the reaction raw materials account for 6~12% of solution gross weight, 2, the mol ratio of 4-diaminophenol hydrochloride and terephthalic acid is (2.1~3.0): 1; c, add molar mass then and be 5~10g of reaction raw material / mol of SnCl 2 Reductant, heat up to 140-210°C, react at this temperature for 2-4 hours to obtain the reaction product; d, the reaction product is neutralized, washed with water, suction filtered, and dried to obtain the initial product; e, the initial product is extracte...
specific Embodiment approach 2
[0010] Specific embodiment two: the p-phenylene-2 of the present embodiment, 2'-bis (5-aminobenzoxazole) is prepared like this: a, under inert gas protection atmosphere, phosphoric acid and P 2 o 5 Mix and configure polyphosphoric acid solution, wherein P in the solution 2 o 5 The concentration is controlled between 82.5% and 83.5%; b. Control the temperature at 60-120°C, add 2,4-diaminophenol hydrochloride and terephthalic acid reaction raw materials to the polyphosphoric acid solution, wherein the reaction raw materials account for 6~12% of solution gross weight, 2, the mol ratio of 4-diaminophenol hydrochloride and terephthalic acid is (2.1~2.5): 1; c, add molar mass then and be 5~8g of reaction raw material / mol of SnCl 2 Reductant, heat up to 140-210°C, react at this temperature for 3-4 hours to obtain the reaction product; d, the reaction product is neutralized, washed with water, suction filtered, and dried to obtain the initial product; e, the initial product is e...
specific Embodiment approach 3
[0011] Specific embodiment three: p-phenylene-2,2'-bis(5-aminobenzoxazole) of the present embodiment is prepared in this way: a, under nitrogen protection, add 71.32g in a 500mL four-necked bottle 85% by weight phosphoric acid and 121.74g P 2 o 5 Make polyphosphoric acid solution; b, cool down to 60°C, add 20.69g (0.105mol) 2,4-diaminophenol hydrochloride and 8.31g (0.05mol) terephthalic acid into the polyphosphoric acid solution; C, then add 0.5g tin protochloride again, be warming up to 210 ℃, react at this temperature for 4 hours, obtain reaction product; d, after reaction product is neutralized by sodium carbonate aqueous solution, suction filtration, dry, obtain initial product; e The initial product was extracted with ethanol and water by Soxhlet respectively, and dried in vacuum to finally obtain bright yellow p-phenylene-2,2'-bis(5-aminobenzoxazole) powder with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com