Amorphous cephalosporin
An amorphous, cefathiamidine technology, applied in the directions of antibacterial drugs, organic chemistry, organic active ingredients, etc., can solve the problems of increasing freeze-drying cost, prolonging freezing and drying time, etc., to achieve industrialization cost advantage and finished product clarity. Good, low solvent residue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0044] Example 1: Preparation of lyophilized cefathiamidine.
[0045]Take by weighing 120 grams of cefathiamidine, add 600ml of pre-cooled water for injection, weigh 0.6g of activated active C after dissolving, decolorize 10mm in an ice bath, and then sterile filter, and the sterile filtrate is placed in a lyophilizer (medical LGJ- LC type freeze dryer), freeze, heat, sublimate and dry according to the freeze-drying curve of cefathiamidine. The obtained white cefathiamidine powder had a content of 95.8%, related substances 1.08%, water content 1.8%, methylene chloride solvent residue 0.01% (pharmacopoeia standard less than 0.06%), and other indicators met the pharmacopoeia standard.
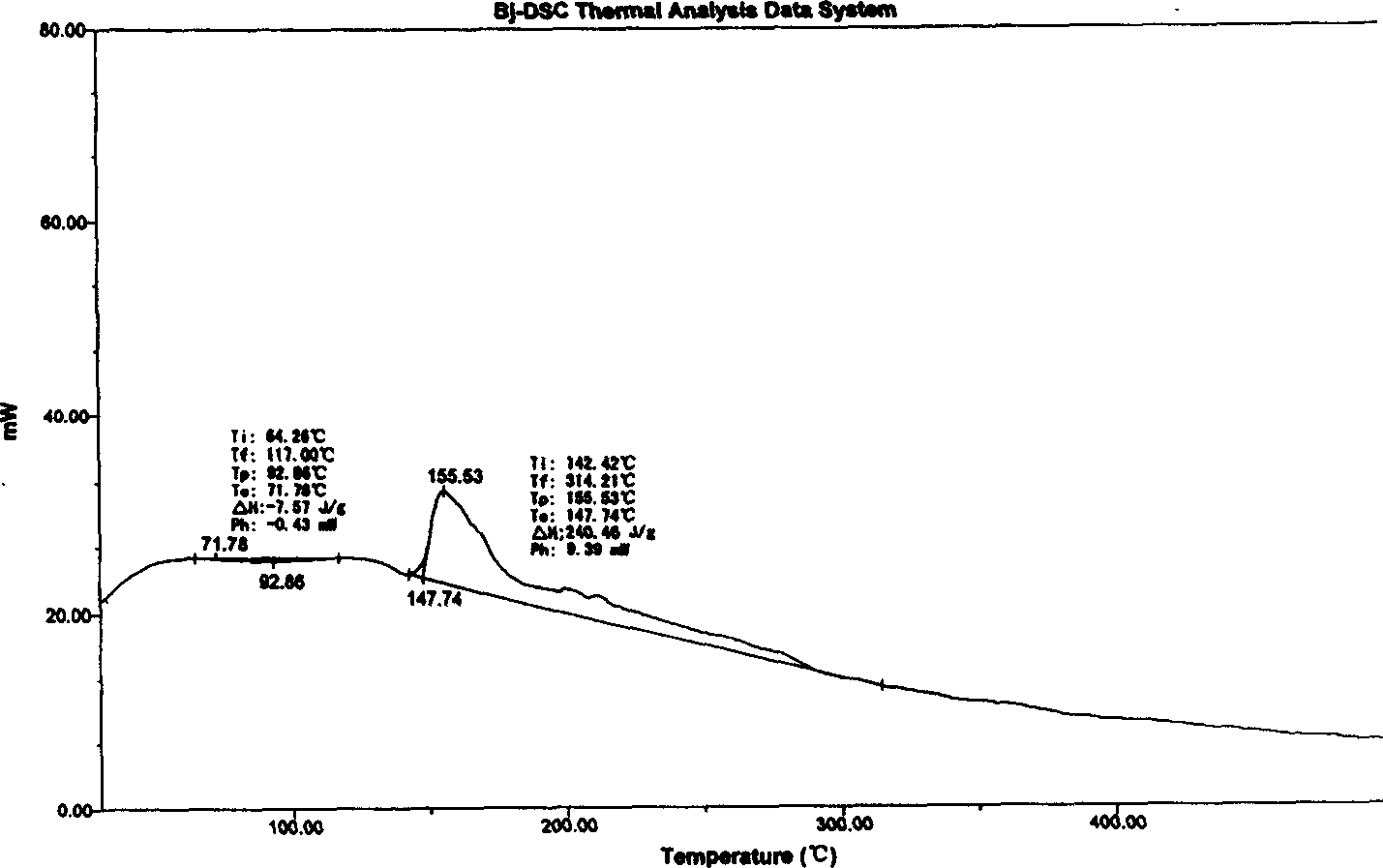
[0046] Differential scanning calorimetry (DSC) curve, it is measured that it exotherms at 147.74 ° C, and its melting decomposition point measured by a melting point instrument is 127 ° C,
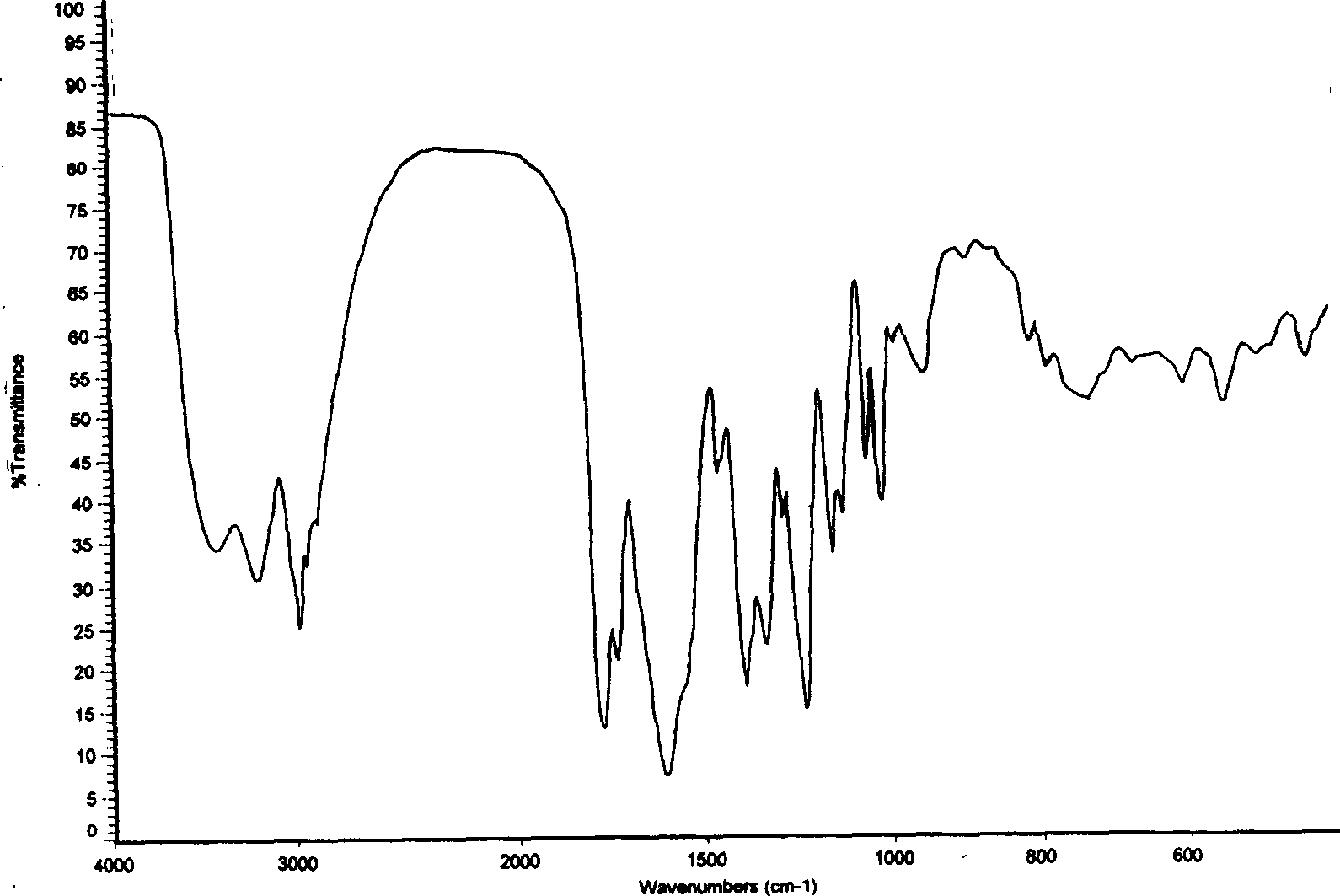
[0047] Infrared absorption spectrum of characteristic functional groups (KBr, cm -1 ) are: 1231.8, 1339....
example 2
[0050] Example 2, the preparation of lyophilized cefathiamidine
[0051] Configuration concentration is 60% cefathiamidine aqueous solution, add 0.4gC decolorization, other conditions are the same as example 1, obtain off-white powder, its content 96.02%, related substance 1.10%, moisture 1.7%, dichloromethane solvent residual 0.01%, other The indicators meet the Pharmacopoeia standards.
[0052] Differential scanning calorimetry (DSC) curve, it is measured that it is exothermic at 147.20 ° C, and its melting point is 127 ° C as measured by the melting point instrument.
[0053] Infrared absorption spectrum of characteristic functional groups (KBr, cm -1 ) are: 1231.2, 1338.6, 1392.1, 1607.9, 1774.7.
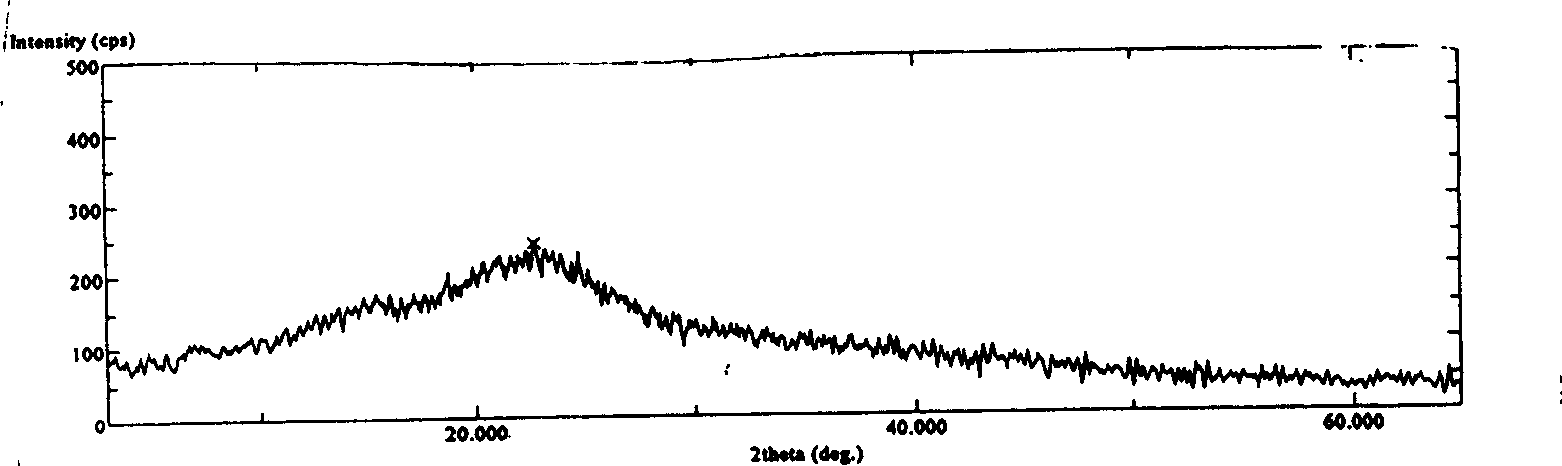
[0054] X-ray diffraction patterns, proved to be amorphous powder.
[0055] The dissolution rate was measured as in Example 1, and the lyophilized powder was dissolved in 4.3 seconds.
example 3
[0056] Example 3, the preparation of lyophilized cefathiamidine
[0057] A 10% cefathiamidine aqueous solution was prepared, and 0.2 g of C was added for decolorization, and other conditions were the same as in Example 1 to obtain a white powder.
[0058] According to the differential scanning calorimetry (DSC) curve, it was measured that it exothermic obviously at 146.9°C, and the melting point instrument showed that its melting decomposition point was 126~127°C.
[0059] Infrared absorption spectrum of characteristic functional groups (KBr, cm -1 ) are: 1231.9, 1339.5, 1393.9, 1774.6.
[0060] X-ray diffraction patterns, proved to be amorphous powder.
[0061] The dissolution rate was measured as in Example 1, and the lyophilized powder was dissolved in 5 seconds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com