Pharmaceutical composition for sustained or continuous releasing therapeutic active components
An active ingredient, sustained release technology, applied in the direction of drug combinations, organic active ingredients, medical formulations containing active ingredients, etc., can solve the problem of drug release at a meaningful and beneficial rate, and rarely reported drug release with limited water solubility and other problems, to achieve the effects of easy mass production, improved drug release, and easy dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
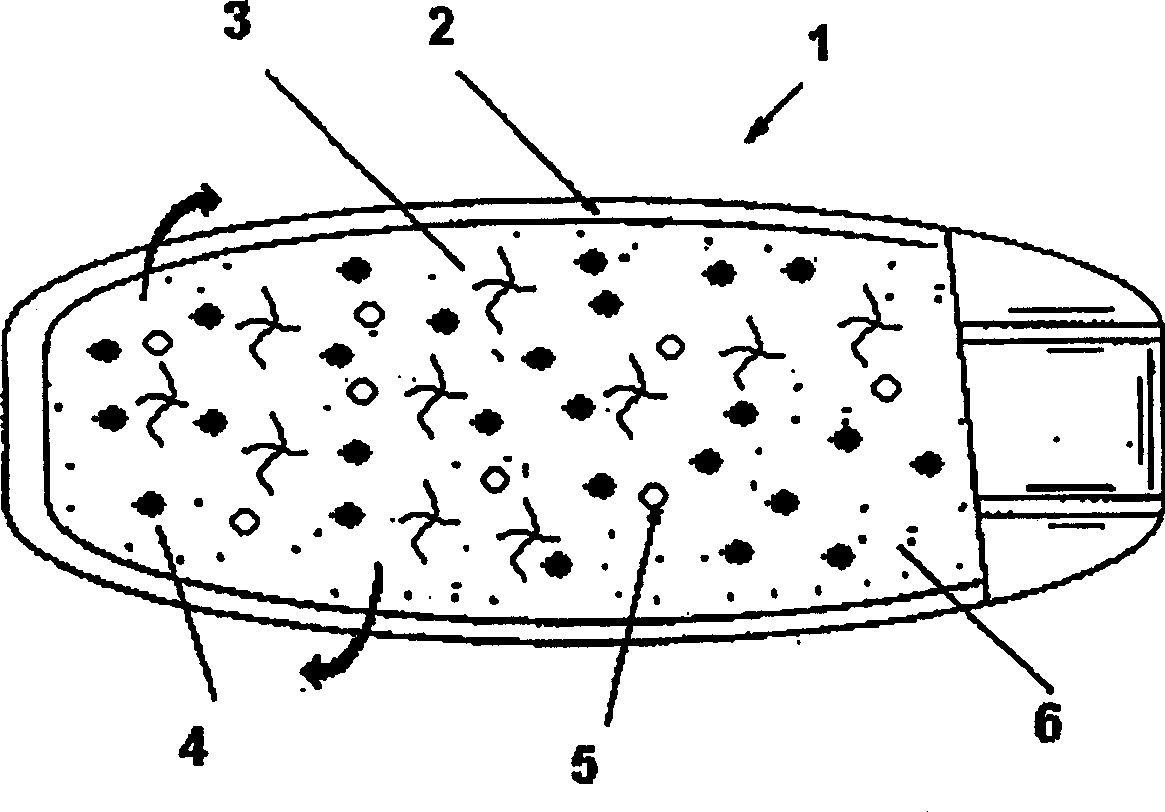
[0073] Another embodiment of the present invention relates to the preparation of coating liquid, and this coating liquid wraps tablet core with the mixture of water-insoluble semipermeable membrane film-forming polymer and water-soluble polymer, and can change semipermeable membrane film-forming polymer and Permeability of the membrane is controlled by the proportion of the membrane-forming polymer.
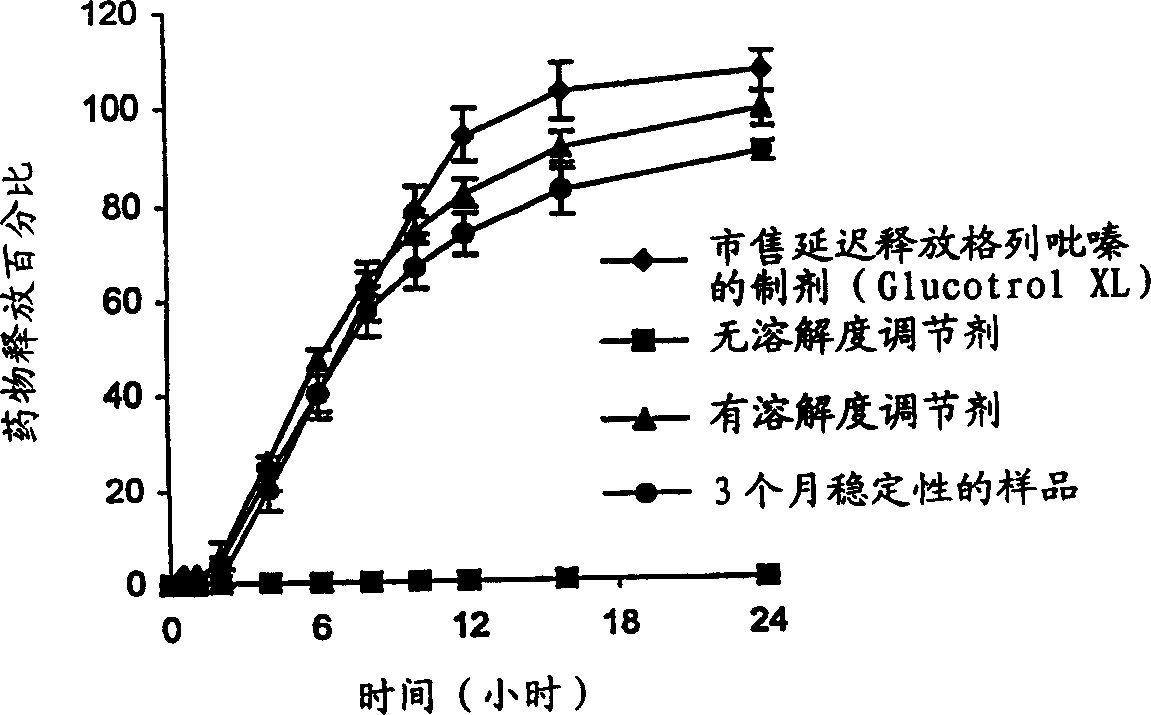
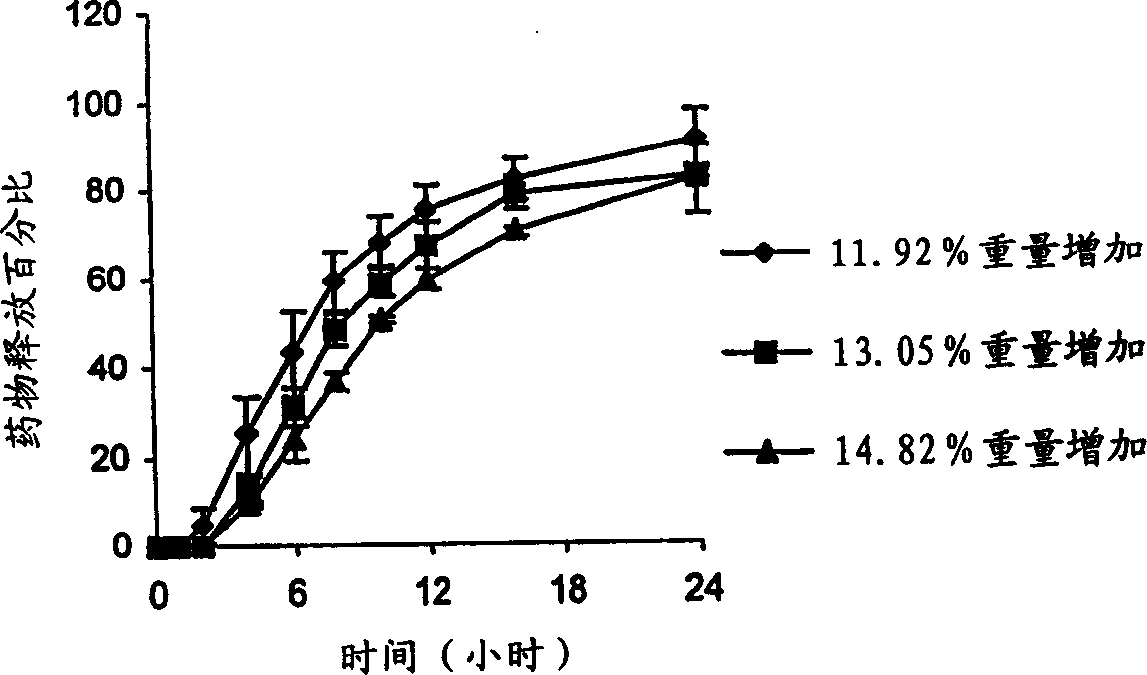
[0074] In general, increasing the concentration of water-insoluble semipermeable membrane-forming polymers decreases membrane permeability and drug release. On the other hand, increasing the concentration of water-soluble polymers increases membrane permeability and drug release. During operation, the core compartment absorbs aqueous liquid from the surrounding environment via the controlled release membrane. The dissolution of the alkalinizing agent / buffer in direct contact with the therapeutically active ingredient results in an increase in the pH of the microenvironment withi...
Embodiment 1
[0097] Prepare the pharmaceutical composition of delayed-release weakly acidic drug-glipizide as follows:
[0098] Tablet cores containing glipizide are prepared as follows:
[0099] No. Ingredient Weight % g mg / tablet
[0100] 1 Glipizide 2.78 6.95 10.00
[0101] 2 TRIS buffer 48.61 121.53 175.00
[0102] 3 Mannitol 29.89 74.73 107.60
[0103] 4 Sodium chloride 9.72 24.30 35.00
[0104] 5 polyvinylpyrrolidone 5.00 12.50 18.00
[0105] 6 Magnesium stearate 1.50 3.75 5.40
[0106] 7 Talc 2.00 5.00 7.20
[0107] 8 Silica airgel 0.50 1.25 1.80
[0108] TRIS buffer (Loba Chemie, India) was mixed with directly compressible mannitol (Pearlitol SD 200, Roquette, France) and sodium chloride (Loba Chemie, India), and passed through a 30-mesh sieve (British Standard Sieve, BSS). Glipizide was mixed with a part of the material obtained above, and passed through a 30-mesh sieve (BSS) after mixing. Mix for 10 minutes and add polyvinylpyrrolidone (Plasdone K 29 / 32, ISP, USA) to the...
Embodiment 2
[0123] The method for preparing as embodiment 1 has the tablet core of the glipizide of following composition:
[0124] No. Ingredient Weight % g mg / tablet
[0125] 1 Glipizide 2.78 2.78 10.00
[0126] 2 TRIS buffer 48.61 48.61 175.00
[0127] 3 Mannitol 30.39 30.39 109.40
[0128] 4 Sodium chloride 9.72 9.72 35.00
[0129] 5 polyvinylpyrrolidone 5.00 5.00 18.00
[0130] 6 Magnesium stearate 1.00 1.00 3.60
[0131] 7 Talc 2.00 2.00 7.20
[0132] 8 Silica airgel 0.50 0.50 1.80
[0133] 100 of the tablets were mixed with 350 grams of filled tablets (filled tablets were prepared with a 7.00 mm round concave punch and contained microcrystalline cellulose, starch, calcium hydrogen phosphate, magnesium stearate, and silica gas Gel) are placed together in the laboratory grade 10 porous applicator, and are coated with a coating solution with the following components:
[0134] No. Ingredient Weight % Grams
[0135] 1 Cellulose acetate 2.58 65.00
[0136] 2 Triacetin 0.26 6.50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com