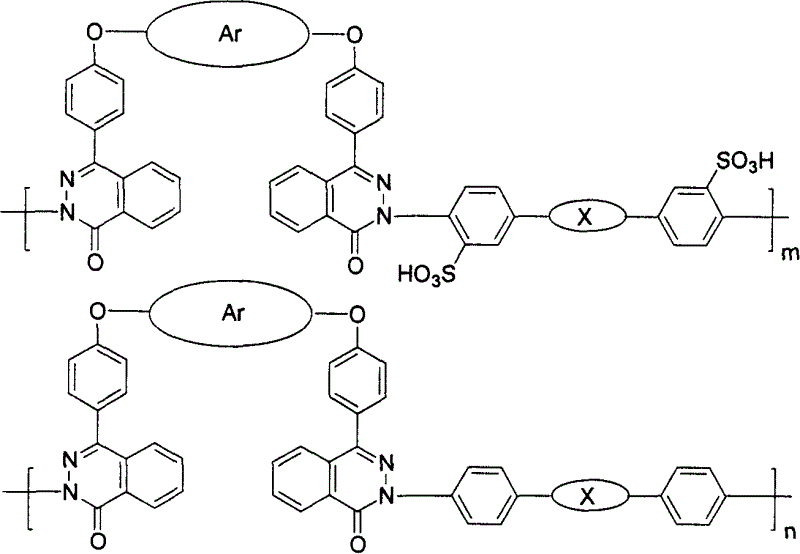
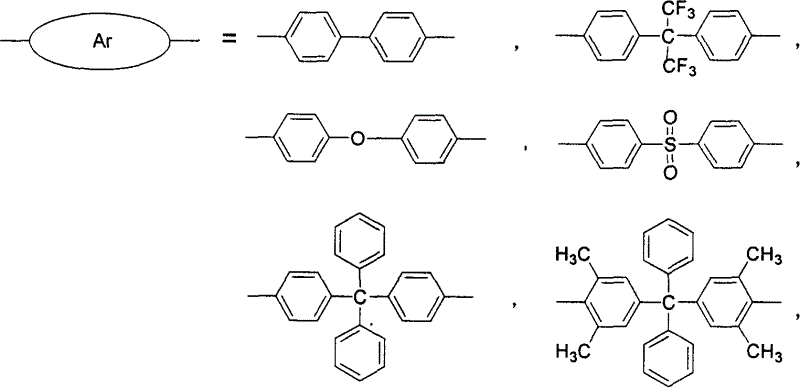
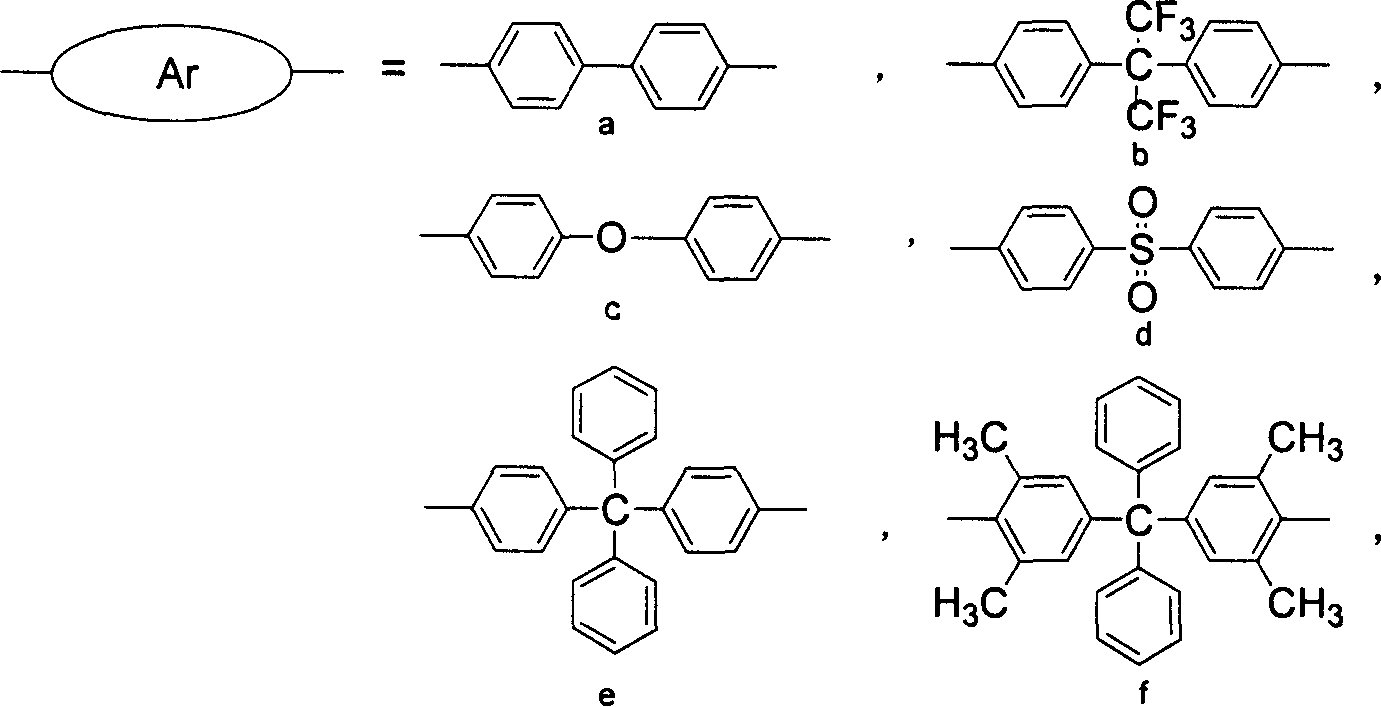
Atactic sulfonated high polymer containing dual diaza naphthone and synthesis and application thereof
The technology of bis-naphthyridine and naphthyridine is applied in the field of random sulfonated high polymers, and can solve the problems of high preparation cost, low alcohol resistance coefficient, complex synthesis route, etc., and achieves solving cost problems, Effects of chemical, physical and mechanical properties on toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0045]Embodiment 1-1, polymer 1aA 80 Synthesis:
[0046] With 0.627g (1mmol) bisphenol-like monomer 2a containing bisdiazinone, 0.338g (0.8mmol) sulfonated dihalogenated monomer 3A, 0.044g (0.2mmol) dihalogenated monomer 4A, 0.194g ( 1.4mmol) of anhydrous potassium carbonate, 5mLDMSO and 6mL toluene were added to a three-necked flask, under the protection of nitrogen, the temperature was raised to 150°C, kept under magnetic stirring for 2 hours, the generated water was taken out by toluene, and then the temperature was raised to 175°C, and the reaction was carried out for 20 hours, cooled, poured the reactant into 100mL methanol-water mixed solution with a volume ratio of 1:1 under constant stirring, filtered, washed three times with distilled water, and dried in vacuum at 80°C for 24 hours to obtain 0.925g of polymer 1aA 80 , yield 96%.
Embodiment 1-2
[0047] Embodiment 1-2, polymer 1aA 60 Synthesis:
[0048] With 0.627g (1mmol) bisphenol-like monomer 2a containing bisdiazinone, 0.253g (0.6mmol) sulfonated dihalogenated monomer 3A, 0.087g (0.4mmol) dihalogenated monomer 4A, 0.194g ( 1.4mmol) of anhydrous potassium carbonate, 5mLDMSO and 6mL toluene were added to a three-necked flask, under the protection of nitrogen, the temperature was raised to 150°C, kept under magnetic stirring for 2 hours, the generated water was taken out by toluene, and then the temperature was raised to 175°C, and the reaction was carried out for 20 hours, cooled, poured the reactant into 100mL methanol-water mixed solution with a volume ratio of 1:1 under constant stirring, filtered, washed three times with distilled water, and dried in vacuum at 80°C for 24 hours to obtain 0.908g of polymer 1aA 60 , yield 98%.
Embodiment 1-3
[0049] Embodiment 1-3, polymer 1aA 40 Synthesis:
[0050] With 0.627g (1mmol) bisphenol-like monomer 2a containing bisdiazinone, 0.169g (0.4mmol) sulfonated dihalogen monomer 3A, 0.131g (0.6mmol) dihalogen monomer 4A, 0.194g ( 1.4mmol) of anhydrous potassium carbonate, 5mLDMSO and 6mL toluene were added to a three-necked flask, under the protection of nitrogen, the temperature was raised to 150°C, kept under magnetic stirring for 2 hours, the generated water was taken out by toluene, and then the temperature was raised to 175°C, and the reaction was carried out for 20 hours, cooled, poured the reactant into 100 mL of methanol-water mixed solution with a volume ratio of 1:1 under constant stirring, filtered, washed three times with distilled water, and dried in vacuum at 80°C for 24 hours to obtain 0.825 g of polymer 1aA80, yield 93 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com