Method for screening acceptor blocker for controlling prawn virus infection
A receptor blocker and virus infection technology, applied in antiviral agents, biochemical equipment and methods, pharmaceutical formulations, etc., to speed up the screening cycle and reduce the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
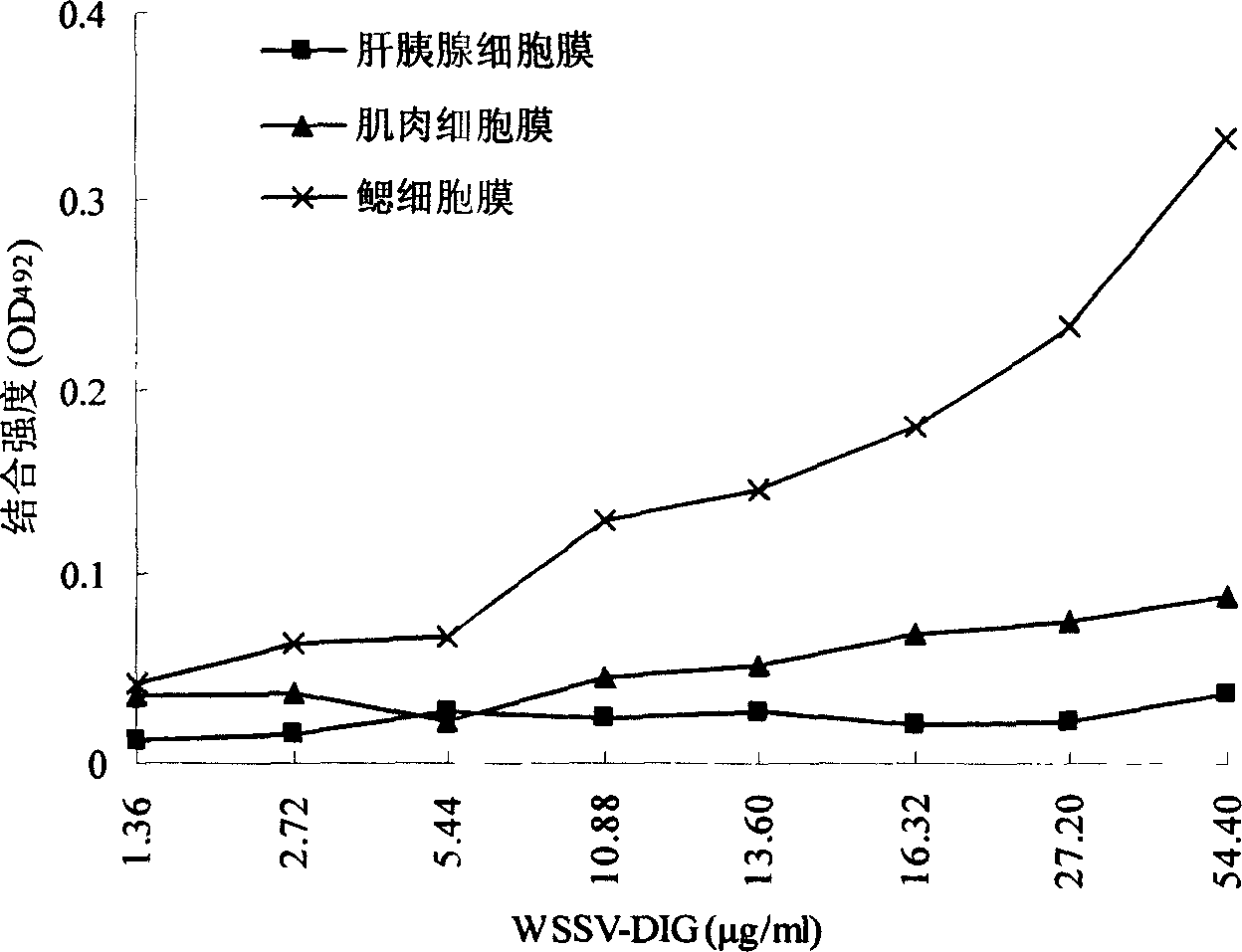
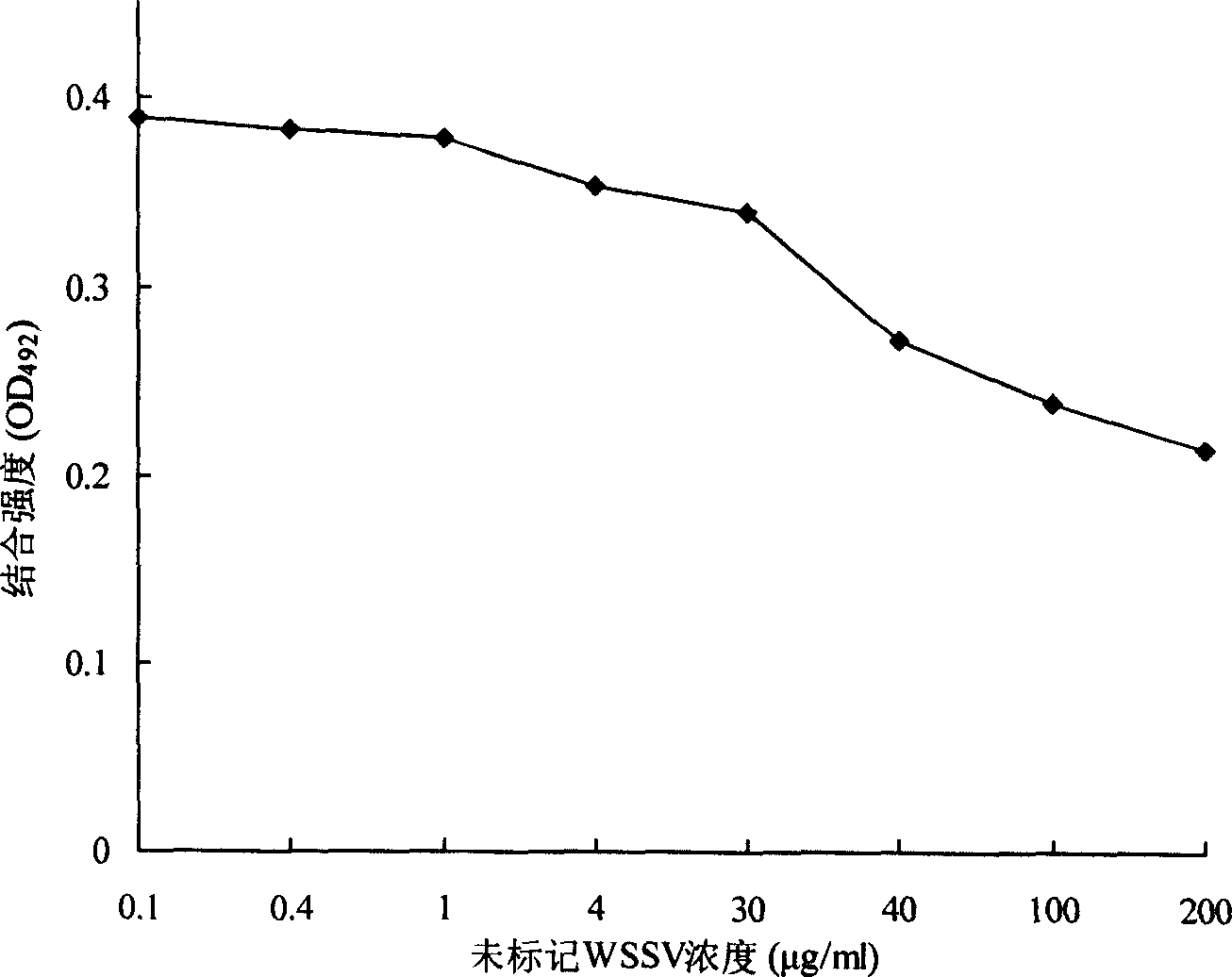
[0036] 1. Example 1: Analysis of specific binding between BCMP and WSSV
[0037] 1.1 WSSV purification
[0038] 1.1.1 Collect shrimps with leukoplakia, weigh 5 g of cephalothorax without hepatopancreas, add 5 times the volume of PPB pre-cooled at 4°C (short for "Physiological Buffer for Prawns", containing: NaCl, 22.0 g / L; K 2 SO 4 , 1.1g / L; CaCl 2 ·H 2 O, 1.9g / L; MgSO 4 ·7H 2 O, 1.6g / L; histidine hydrochloride, 5.0g / L; pH 6.5), homogenize in an ice bath at 20,000r / min for 3-5 times, each time for 5s.
[0039] 1.1.2 Centrifuge at 4,500×g for 15 minutes at 4°C, add sucrose to the supernatant to a concentration of 30% (W / W), and centrifuge at 30,000×g for 2 hours at 4°C.
[0040] 1.1.3 The pellet was resuspended with 30% (W / W) sucrose, spread on a 33%-62% (W / W) sucrose gradient, centrifuged at 60,000×g at 4°C for 4 hours, and carefully collected visible virus bands with a pipette.
[0041] 1.1.4 Add 5 times the volume of PPB to the collected virus zone solution to dilute ...
Embodiment 2
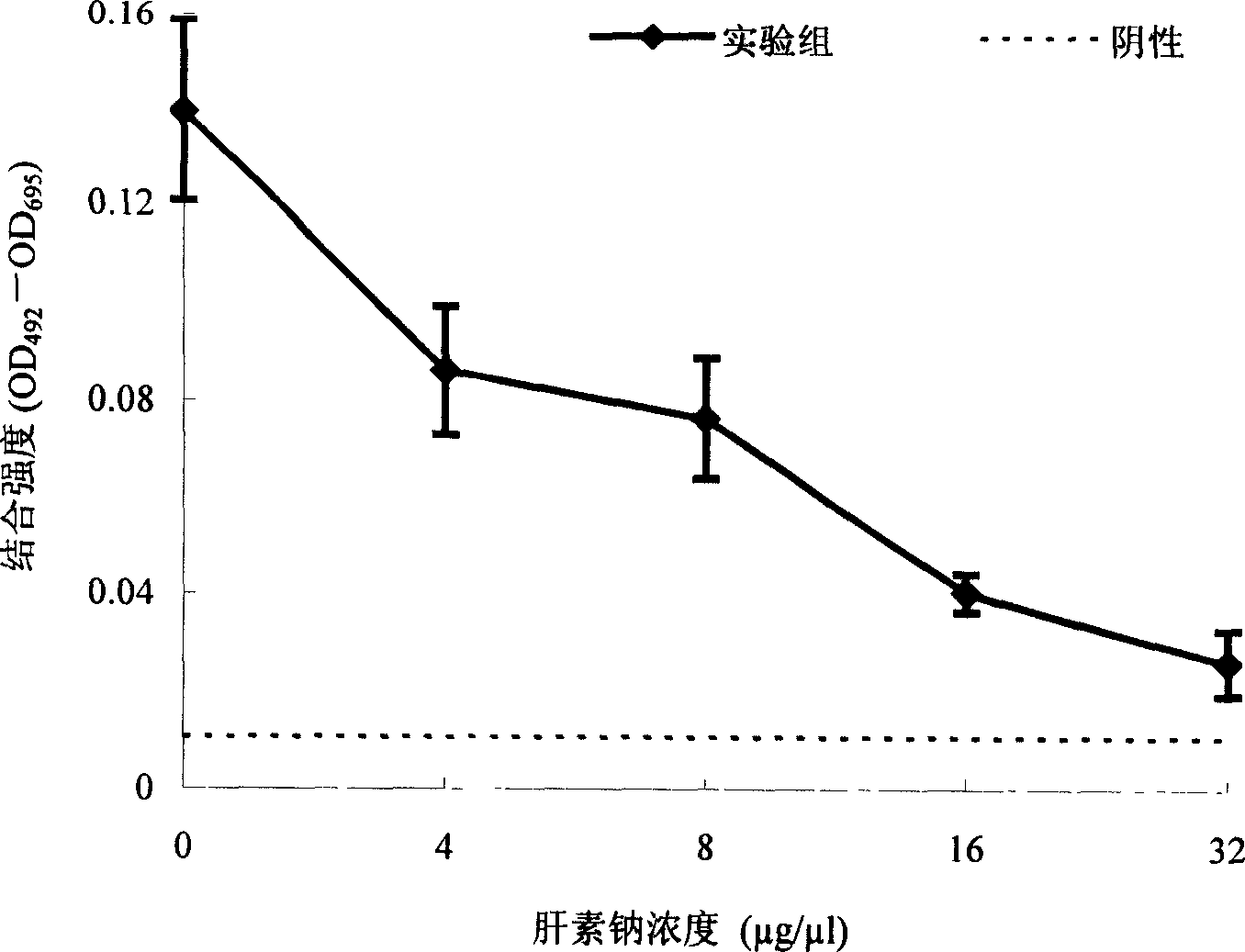
[0070] 2. Example 2: Screening of polysaccharides that inhibit the binding of WSSV and BCMP
[0071] 2.1 Binding inhibition analysis of heparin sodium:
[0072] 2.1.1 Treatment of DIG-WSSV by heparin sodium:
[0073] - Dilution of heparin sodium: Dilute heparin sodium with an original concentration of 0.16 g / ml in dHO in an Eppendorf tube 2 O was diluted into 4 gradients of heparin sodium by 2-fold dilution. Concentrations are: 0.08g / ml, 0.04g / ml, 0.02g / ml, 0.01g / ml.
[0074] - Heparin treatment of DIG-WSSV: Add 300 μl of 0.124 μg / μl DIG-WSSV (diluted in MPBS) to four Eppendorf tubes, and mix 120 μl of the above heparin sodium concentration gradient with the DIG-WSSV in the tube. The final concentrations of heparin sodium in each tube were: 32 μg / μl, 16 μg / μl, 8 μg / μl and 4 μg / μl.
[0075] 2.1.2 Coating: After diluting 1.0 μg / μl BCMP with coating solution, coat 1.5 μg / 100 μl / well on a 96-well microtiter plate, overnight at 4°C.
[0076] 2.1.3 Plate washing: The next day, ...
Embodiment 3
[0108] 3. Example 3: Infection experiment to verify the anti-infection effect of heparin sodium on WSSV treatment
[0109] 3.1 Experimental shrimp and grouping
[0110] 3.1.1 Litopenaeus vannamei is provided by Qingdao Jiaonan Farm, 9-12cm in size, with an average of 14.8g / tail.
[0111] 3.1.2 After the experimental shrimps were inflated for 3 days and stabilized in the laboratory, the healthy and lively ones were selected and divided into two groups. The P group was the WSSV infection group, which was used as the infection control group. The H group was the heparin sodium experimental group, 12 fish / group.
[0112] 3.2 Virus seed treatment
[0113] 3.2.1 Take 20 g of cephalothorax (with hepatopancreas removed) diseased material of prawns infected by WSSV, add 2% NaCl, and homogenize in an ice bath.
[0114] 3.2.2 The homogenate was centrifuged at 4,500×g for 20 minutes at 4°C, and the sedimentation was repeated 3 times.
[0115] 3.3.3 The supernatants were combined, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com