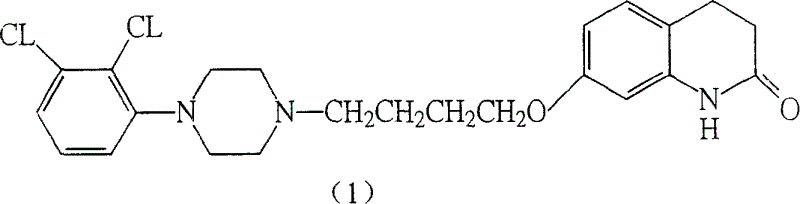Method for preparing aripiprazole and its intermediate
A piperazine and Chinese-style technology, applied in the field of preparation of antipsychotic drug aripiprazole, can solve the problems of long reaction time, low yield, unfavorable industrial production, etc., and achieve the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
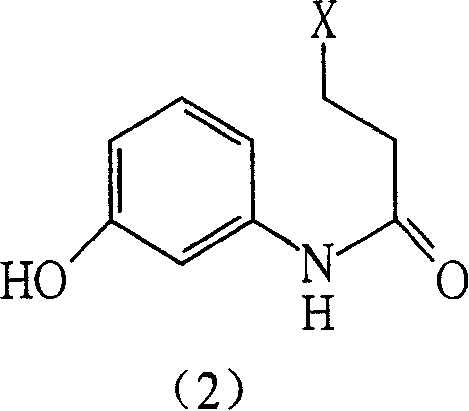
[0071] Stir and dissolve 100g m-aminophenol, 100g sodium bicarbonate, 400ml methanol, and 77.0ml water, and cool in an ice-salt bath. Add 96.0ml CLCH dropwise 2 CH 2 COCL, dropwise, react below 30°C for 3-4 hours, adjust the pH to 2 with concentrated hydrochloric acid, stir for 0.5 hours, add water, filter, and dry to obtain 145 g of the compound of formula (II) as white crystals. Yield 79%, melting point 130-132°C. Purity check: TLC analysis: ethyl acetate: absolute ethanol = 20:1, observed under ultraviolet light. Rf = 0.4.
[0072] IR (KBr cm -1 ): 1668.03, 1619.16, 1545.54, 1451.74, 1274.16, 1236.99, 873.87, 684.67
Embodiment 2
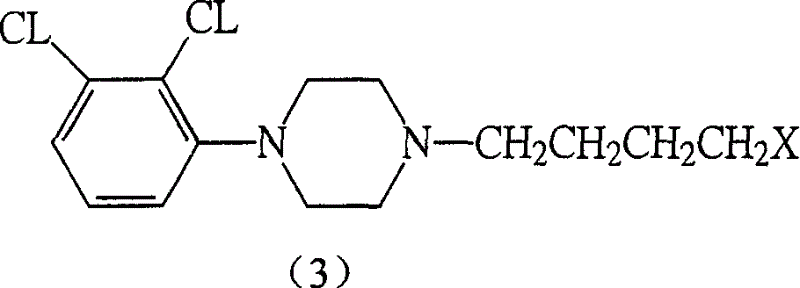
[0074] Add 138g of 1-(2,3-dichlorophenyl)piperazine and 1600ml of DMF to 1.4 dibromobutane for reaction, dilute with water, separate the organic layer, extract the aqueous layer with ethyl acetate, combine the organic layers, and concentrate under reduced pressure for at least amount, add acetone until the residue dissolves, add petroleum ether to precipitate, filter, and dry to obtain a brown powder, which is subjected to silica gel column chromatography to obtain 124 g of the compound of formula (III) as white needle crystals. The melting point is 120-121°C. Yield: 33.9%, purity check: TLC analysis: ethyl acetate:petroleum ether=1:2, observed under ultraviolet light. Rf = 0.7.
[0075] IR (KBr cm -1 ): 1525.04, 1494.5, 1382.31, 1199.03, 1176.57, 1059.75, 859.05, 788.04, 627.86
Embodiment 3
[0077] Add 100g of compound of formula (II), 150g of sodium iodide, and 1600ml of acetonitrile into a three-necked flask, heat, and reflux the suspension for 0.5h, then add 202.0ml of aqueous solution containing 276g of potassium carbonate, and then add 183g of compound of formula (III) , react at 60° C. for about 1 h, or TLC detects that the raw material point disappears, stop the reaction, add a large amount of water to dilute, precipitate out, filter to obtain 165 g of the compound of formula (IV) as a white powder, and the yield is 68%. Purity check: TLC analysis: ethyl acetate:petroleum ether=1:2, observed under ultraviolet light. Rf = 0.3.
[0078] IR (KBr cm -1 ): 3034.09, 1676.57, 1630.24, 1592.99, 1525.04, 1494.5, 1382.31, 1199.03, 1176.57, 1059.75, 859.05, 788.04, 627.86
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com