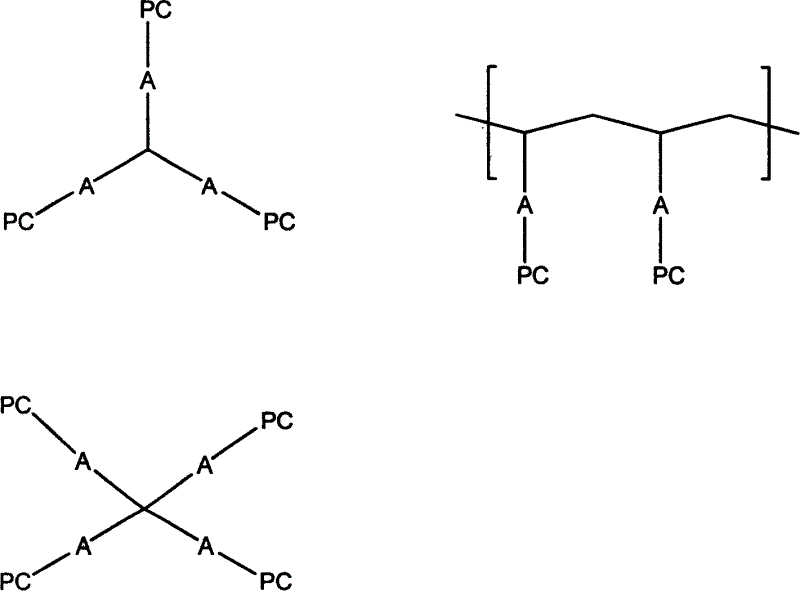
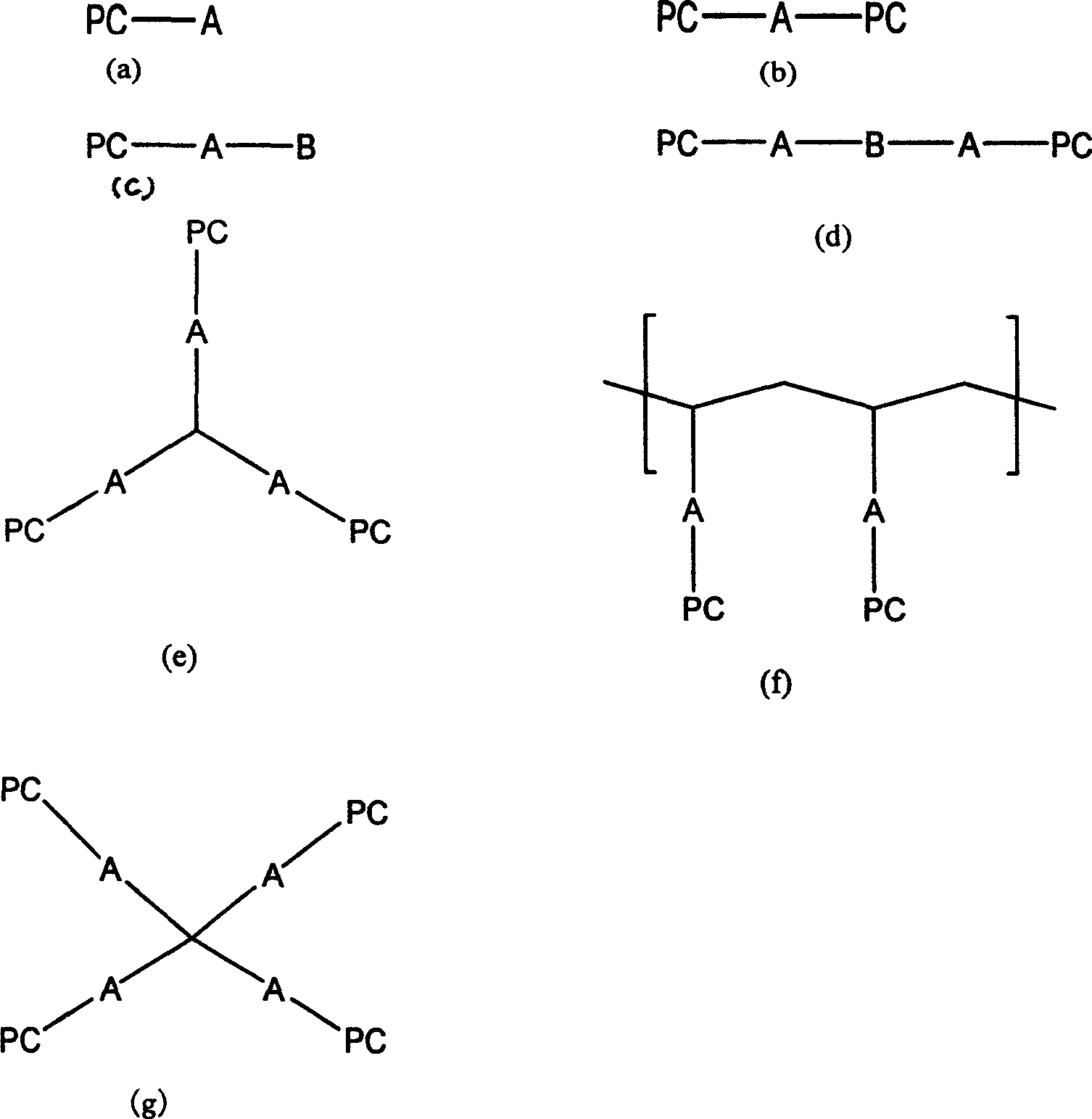
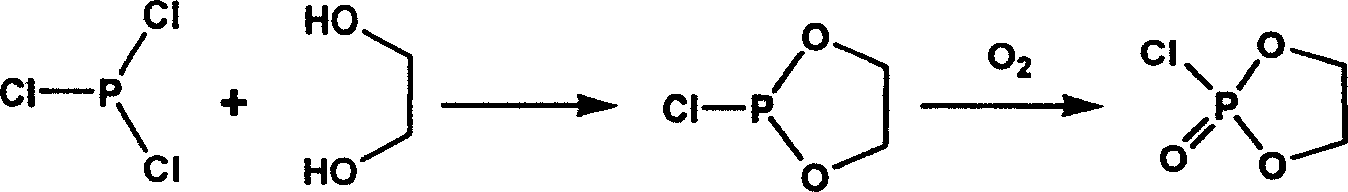
Lipoid bio-degradable polyester and preparation thereof
A technology for degrading polyester and phospholipid, applied in the field of biodegradable polymer materials, can solve the problems of poor drug permeability and slow degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]In a three-neck flask equipped with a constant pressure dropping funnel and a magnetic stirrer, add polycaprolactone with hydroxyl groups at both ends, triethylamine and dry tetrahydrofuran in a ratio of 1:2:2. Stir, use an ice-salt bath to cool the reaction system to -17°C, slowly drop in COP (2-chloro-1,3-dioxophosphoryl) dissolved in a certain amount of dry tetrahydrofuran for 3 hours, then continue to stir for 2 hours . Control the temperature at -17°C. The temperature was naturally raised to room temperature, and the reaction was stirred for 2.5 hours. The triethylamine chloride (in the form of white crystals) was removed by suction filtration, and the solvent THF was removed by rotary evaporation under reduced pressure at 30°C to obtain the white viscous product COP-PCL. Add dry acetonitrile and COP-PCL into a thick-walled single-necked flask with a stopcock, shake well, and then add a certain amount of dry trimethylamine saturated acetonitrile solution. Close t...
Embodiment 2
[0034] In a three-neck flask equipped with a constant pressure dropping funnel and a magnetic stirrer, polyglycolic acid (PGA), triethylamine and dry toluene were added in a ratio of 1:2:2. After stirring, the reaction system was cooled to 0°C with ice-salt mixture, and COP dissolved in a certain amount of dry toluene was slowly added dropwise for 3 hours, and then continued to stir for 3 hours. Control the temperature at 0°C. The temperature was naturally raised to room temperature, and the reaction was stirred for another 2 hours. The chloride of triethylamine (in the form of white crystals) was removed by suction filtration, and the solvent toluene was removed by rotary evaporation under reduced pressure at 40° C. to obtain the white viscous product COP-PGA. Add dry acetonitrile and COP-PGA into a thick-walled single-necked flask with a stopcock, dissolve, shake well, and then add a certain amount of acetonitrile saturated solution of dry trimethylamine. Close the plunger...
Embodiment 3
[0036] In a three-neck flask equipped with a constant-pressure dropping funnel and a magnetic stirrer, add dry polylactic acid (PLA) powder, triethylamine and dry dioxane whose surface was ammonolyzed with hexamethylenediamine for 2 hours in proportion. Stir to keep the system in a suspended state, use ice-salt mixture to cool the reaction system to -10°C, slowly drop in excess 100% COP dissolved in dry dioxane for 2 hours, and then continue to stir for 2 hours. Control the temperature at -10°C. The temperature was naturally raised to room temperature, and the reaction was stirred for another 2 hours. Filtration and vacuum removal of triethylamine chloride yielded white product COP-PLA. Add dry acetonitrile and COP-PLA into a thick-walled single-necked flask with a stopper, dissolve, shake well, and pass excess dry trimethylamine into it. Close the plunger, put it in a water bath, open the plunger, and heat until the residual trimethylamine is driven into the concentrated su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com