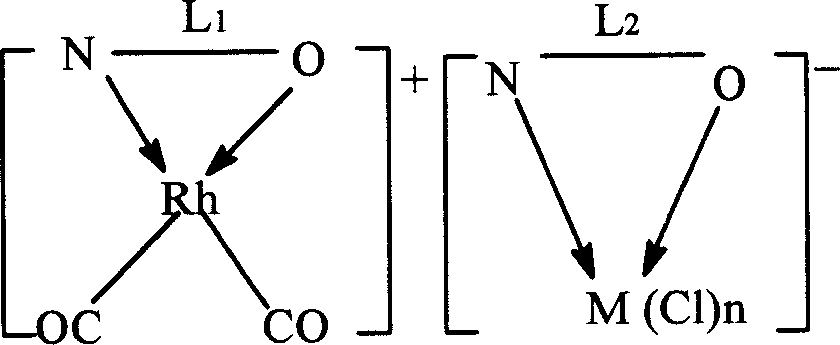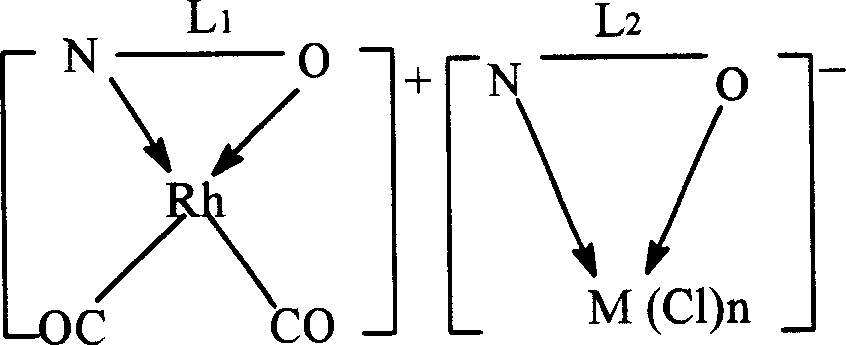Double active species catalyst and its application
A catalyst and dual-activity technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, carbon monoxide reaction to prepare carboxylic acid, etc., can solve the problems of water gas reaction acceleration, consumption, and increased equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation method of the transition metal complex of the anion part of the catalyst takes the nickel complex of sodium pyridine-3-formate as an example.
[0042] Weigh 0.02 mol of pyridine-3-carboxylic acid and NaOH, dissolve them in 2 mol of water, heat to 70°C for 1 h, cool, precipitate with excess acetone, and dry to obtain sodium pyridine-3-carboxylate.
[0043] Weigh 0.01mol of sodium pyridine-3-formate and dissolve it in a mixture of 1mol of methanol and 0.5mol of water, add 0.01mol of NiCl 2 , heated to reflux under stirring for 1h, cooled, precipitated with acetone, and dried to obtain pyridine-3-formic acid sodium-nickel complex.
[0044] Iron, Copper, Cobalt and Ru Complexes. The nickel, iron, copper, cobalt and ruthenium complexes of sodium aminoacetate, sodium 3-aminopropionate, sodium 4-aminobutyrate, sodium anthranilate, sodium m-aminobenzoate and sodium anthranilate were prepared by the above method.
Embodiment 2
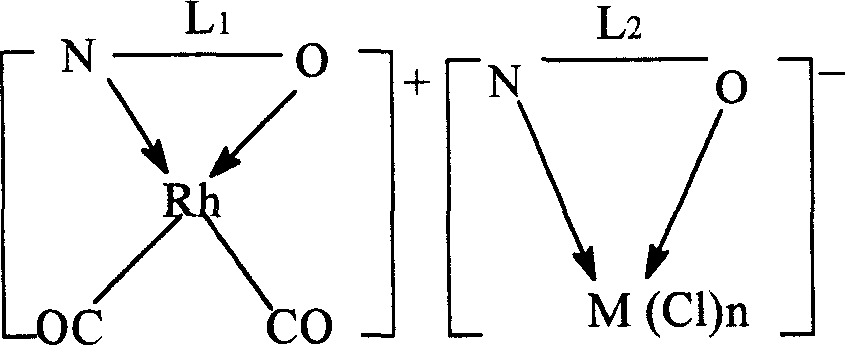
[0046] The preparation of the dual-active species catalyst is an example of a pyridine-3-formic acid rhodium-pyridine-3-formic acid nickel dual-active species catalyst.
[0047] Weigh 0.02mol of pyridine-3-carboxylic acid and 0.01mol of [Rh(CO) 2 Cl] 2 Dissolve in 3mol of methanol, stir and react at 70°C for 1h, then add methanol-water mixture containing 0.01mol of pyridine-3-formic acid sodium-nickel complex (the molar ratio of methanol to water is 2:1), continue at 70°C The reaction was stirred for 1 h. After cooling, it was precipitated with ether and filtered. Wash twice with methanol water (2:1 mol) mixed solution at 0°C, and dry at room temperature to constant weight to obtain brown pyridine-3-carboxylate rhodium-pyridine-3-carboxylate nickel dual-active species catalyst.
[0048] Adopt above-mentioned method to prepare the cobalt, iron, copper and ruthenium of pyridine carboxylic acid listed in embodiment 1 and aminocarboxylate nickel, cobalt, iron, copper dual activ...
Embodiment 3
[0052] Add 0.3 g of rhodium pyridine-2-carboxylate-nickel pyridine-3-carboxylate catalyst, 1.24 mol of methanol, 0.87 mol of acetic acid, and 0.24 mol of methyl iodide into a 250 ml zirconium autoclave, heat up to 150 ° C after feeding CO, and maintain the reaction pressure 4.0MPa, stirring speed 500 rpm, reaction time 30min. The conversion rate of methanol is 100%, the content of methyl acetate is 0.02 mol, the increment of acetic acid is 1.19 mol, and the space-time yield of acetic acid is 20.7 mol AcOH / L·h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com