Method of screening out model for preparing hydrolase inhibitor of adenosine homocysteine
A technology for screening inhibitors and cysteine, which is applied in biochemical equipment and methods, microbial measurement/inspection, etc., can solve the problems of impracticability, complicated process, enzyme waste, etc., and achieve easy preservation, maintenance of activity, and stability sex increasing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
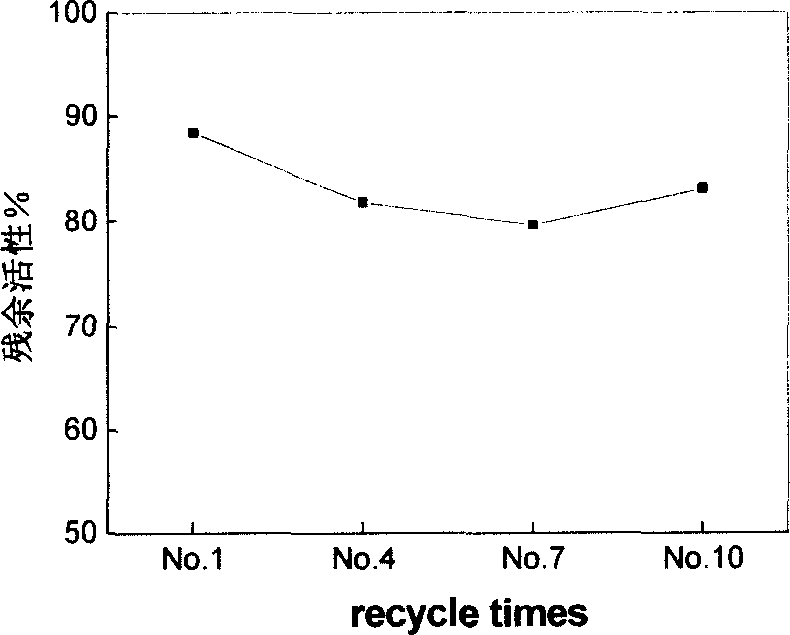
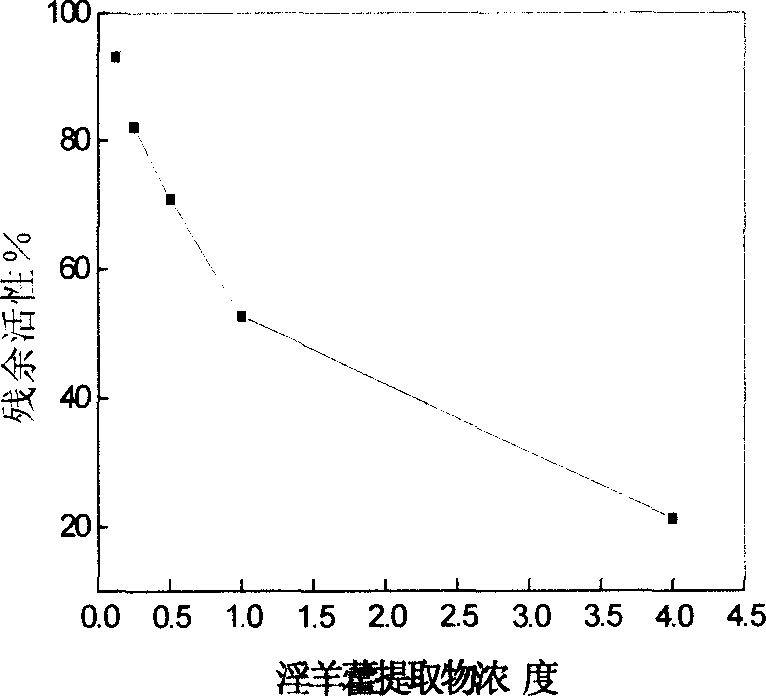
Examples
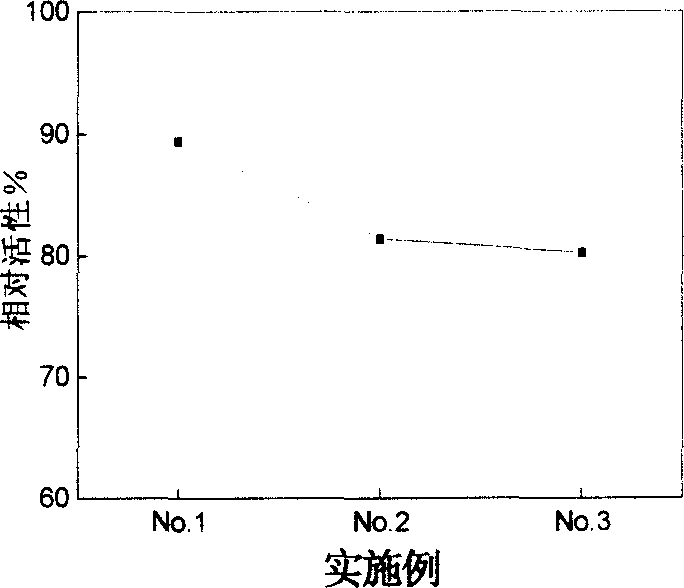
Embodiment 1
[0023] 1) Coupling: The reaction was carried out at 4°C, and all reagents used were pre-cooled at 4°C. Take 1 mL of NHS-activated Sepharose 4B preserved in isopropanol, centrifuge at 3000 rpm for 5 min, discard the supernatant, and replace with 1 mmol·L -1 Hydrochloric acid and pH7.4 10mmol L -1 Na 2 HPO 4 / NaH 2 PO 4 , 1mmol·L -1 EDTA buffer was washed 3 times, and 0.5 mg·mL was added -1 Adenosyl homocysteine hydrolase 1mL, mix well, shake at 200rpm for 6 hours. After the reaction is completed, centrifuge at 3000rpm, and combine the supernatant and the washing solution obtained in the blocking step to measure the protein concentration (Bradford method). The enzyme protein concentration of the immobilized enzyme=the total concentration of added protein-the protein concentration in the supernatant.
[0024] 2) Blocking: the obtained precipitate was washed with 50mmol·L -1 500 μL Tris-HCl buffer solution of pH 7.4 was blocked overnight, centrifuged at 3000 rpm, and w...
Embodiment 2
[0028] 1) Coupling: The reaction was carried out at 4°C. Take 5 mL of NHS-activated Sepharose 4B preserved in isopropanol, centrifuge at 3000 rpm for 5 min, and precipitate with 1 mmol L -1 10mmol·L of hydrochloric acid and pH7.4 -1 Tris-HCl, 1mmol L -1 EDTA buffer was washed 3 times, and 0.5 mg·mL was added -1 Adenosyl homocysteine hydrolase 2mL, shake at 100rpm for 4 hours. After the reaction was completed, centrifuge at 3000rpm, and the protein concentration determination method was the same as in Example 1.
[0029]2) Blocking: the obtained precipitate was washed with 50mmol·L -1 2 mL of Tris-HCl buffer at pH 7.4 was blocked overnight, centrifuged at 2000 rpm, and washed with 50 mmol·L -1 Tris-HCl at pH8.0 / 1mmol·L -1 NaCl and 1mmol L -1 Two buffer solutions of HAc-NaAc at pH 4.5 were washed alternately for 5 times to obtain a washing solution.
[0030] 3) Activation: add the final concentration of 0.5mmol L -1 Oxidized coenzyme I (NAD + ) of 10mmol·L -1 ...
Embodiment 3
[0032] 1) The coupling and blocking reactions are the same as in Example 1, and the carrier is NHS-activated Sepharose 6B preserved in isopropanol.
[0033] 3) Activation: add the final concentration of 0.1mmol L -1 Oxidized coenzyme I (NAD + ) of 10mmol·L -1 pH7.4 phosphate buffer solution to a final volume of 1mL, left at room temperature for 3 hours, and then 10mmol·L -1 Phosphate buffer at pH 7.4 was washed until the supernatant had stable absorbance at 280 nm and 340 nm. Consistent with the method for measuring enzyme activity in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com