Flame retardant polyesterpolymer able to dye by using cation pigment,its mfg.method and copolymer fiber using the same
一种阳离子染料、阻燃聚酯的技术,应用在共聚酯纤维领域,能够解决磷含量减少、产生有毒气体、原纱可纺性和物理性能降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
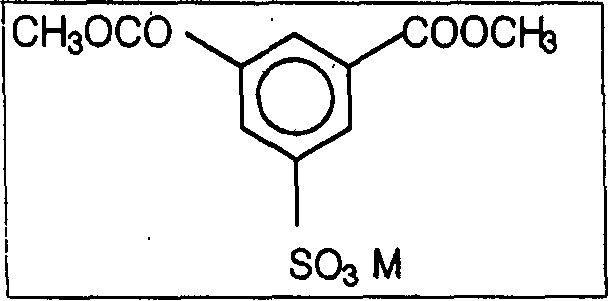
[0064] After dissolving DMS (wherein M is sodium) in the EG in the reactor, wherein the reactor is equipped with a methanol discharge unit so that the content of DMS is 60%, sodium hydroxide (NaOH) is added as a transesterification reaction catalyst to the resulting In solution, it was 5 wt% of DMS to initiate the reaction. When the discharged methanol content exceeds 99% of the theoretical value, the heating is stopped and the reactor is cooled to produce DES.
[0065] The slurry was prepared using DES so that the molar ratio of DES and (TPA+DES) was 1.5, while in this case the molar ratio of EG to all acid components (TPA+DES) was 1.15.
[0066]An alkali oligomer having the same composition as the slurry was stirred in an esterification tank at 255°C, and then the slurry was fed into an esterification reaction tank while the internal temperature of the reactor was kept at 255°C. After the feeding of the slurry was completed, the stirring was continued for 30 minutes, and wh...
Embodiment 2
[0070] After dissolving DMS (wherein M is sodium) in the EG in the reactor, wherein the reactor is equipped with a methanol discharge device so that the content of DMS is 60%, sodium hydroxide (NaOH) as a catalyst for the transesterification reaction is added to the resulting In solution, it was 5 wt% of DMS to initiate the reaction. When the discharged methanol content exceeds 99% of the theoretical value, the heating is stopped and the reactor is cooled to produce DES.
[0071] The slurry was prepared using DES so that the molar ratio of DES and (TPA+DES) was 1.2, while in this case the molar ratio of EG to all acid components (TPA+DES) was 1.15.
[0072] An alkali oligomer having the same composition as the slurry was stirred in an esterification reaction tank at 255°C, and then the slurry was fed into the esterification reaction tank while the internal temperature of the reactor was maintained at 255°C. After the feeding of the slurry was completed, the stirring was conti...
Embodiment 3
[0076] A slurry was prepared such that the molar ratio of EG to TPA was 1.15.
[0077] The molar ratio of DES and (TPA+DES) was kept at 1.5, the DE reaction tank was kept at 255° C. while stirring, and then stirring was continued for 30 minutes after the slurry and DES were sequentially fed into the DE reaction tank. The slurry and DES were supplied in such a manner that their amounts were controlled so that the molar ratio of DES and (TPA+DES) was 1.5, and then the reaction was continued for 30 minutes, whereby the degree of reaction was close to 95.2%.
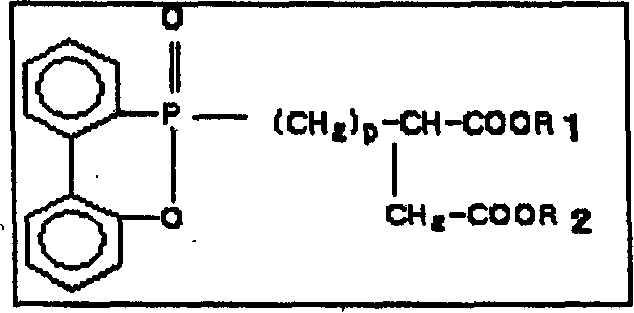
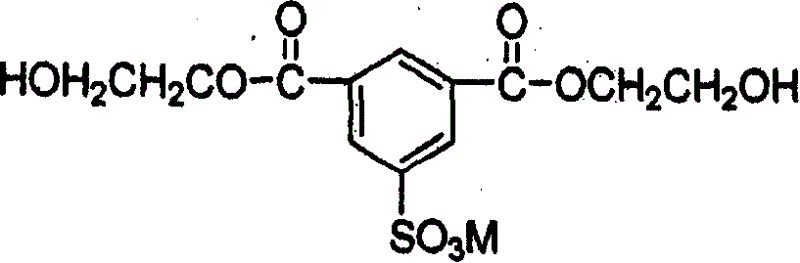
[0078] The resulting product is transferred to the polycondensation tank and will include hydroxyethyl (CH 2 CH 2 OH) as R in chemical formula 1 1 and R 2 The flame retardant is supplied into the polycondensation reaction tank so that the phosphorus atom content contained in the flame retardant is 6100ppm of the polymer, manganese acetate is supplied into the polycondensation reaction tank as a manganese salt so that the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| stretch ratio | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com