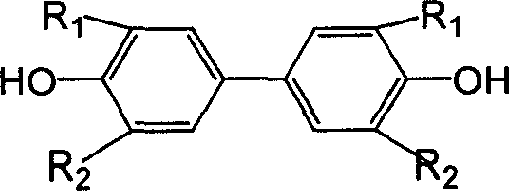
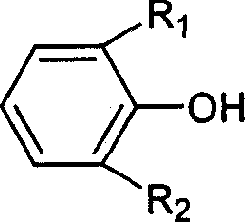
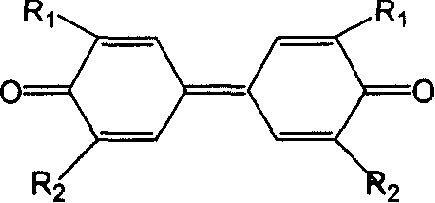
Prepn process of 3,3',5,5'-tetraalkyl-4,4'-diphenol
A technology of biphenyldiphenol and dialkylphenol, which is applied in 3 fields, can solve the problems of poor reaction selectivity, etc., and achieve the effects of less equipment investment, novel synthesis method and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 6.1g 2,6 dimethylphenol (0.05mol), after grinding, add 8.5g AgNO 3 (0.05mol), 50g deionized water, stirred, the temperature was controlled at 0 ° C, reacted for 4 hours, suction filtered, washed with water, then washed with NaOH solution, suction filtered and dried to obtain product 3.2g (0.013mol), yield 52% . Through infrared spectroscopy, 1 H-NMR proved to be 3,3',5,5'-tetramethyl-4,4'-biphendiquinone, and its data are as follows: IR (KBr, v cm -1 )1693, C=O, 1638, C=C, 1380, CH 3 ;exist 1 In the H-NMR chart, 7.700ppm is the chemical shift of H on the double bond in the quinone, 2.135ppm is the chemical shift of H on the methyl group, the integral area ratio is 1:3; the melting point is 215-217°C.
[0027] Take 2.4 g (0.01 mol) of 3,3',5,5'-tetramethyl-4,4'-diphenyldiquinone, add 50 mL of ethanol, and then add 19 g of NaHSO 3 (0.18mol) and 16.8g NaHCO 3 (0.2mol), then 18g of deionized water was added, and the solution was stirred at room temperature for 1 hour....
Embodiment 2
[0029] 3.1g 2,6-dimethylphenol (0.025mol), after grinding, add 54g FeCl 3 .6H 2 O (0.2mol), 40g deionized water, stirred, the temperature was controlled at 20 ° C, reacted for 4 hours, suction filtered, washed with water, then washed with NaOH solution, suction filtered and dried to obtain product 2.0g (0.008mol), yield 63 %.
[0030] Take 1.2 g (0.005 mol) of 3,3',5,5'-tetramethyl-4,4'-biphendiquinone, add 25 mL of ethanol, and then add 11 g of Na 2 S 2 O 3 (0.07mol) and 10gNH 4 NO 3 (0.125mol), then 9g of deionized water was added, and the solution was stirred at room temperature for 1 hour. The solution changed from red to nearly colorless lower layer, and the organic solvent layer was yellow. The mixture was allowed to stand still until the layers were separated, spun off to remove the ethanol, suction filtered, washed with water, and dried in vacuo to obtain 1.1 g (0.0045 mol) of a pale yellow solid with a yield of 92%.
Embodiment 3
[0032] 10.2g 2,6-di-tert-butylphenol (0.05mol), after grinding, add 10.0g copper acetate (0.05mol), 80g deionized water, N 2 Under the protection, stirring, the temperature is controlled at 20 ° C, the reaction is carried out for 4 hours, suction filtration, washing with water, then washing with NaOH solution, suction filtration and drying to obtain 3,3',5,5'-tetra-tert-butyl-4,4' - Biphendiquinone 7.69 g (0.02 mol), yield 63%.
[0033] Take 4.8 g (0.012 mol) of 3,3',5,5'-tetra-tert-butyl-4,4'-diphenyldiquinone, add 50 mL of dichloroethane, and then add 20.9 g of Na 2 S2 O 4 (0.12mol) and 40g Na 2 HPO 4 (0.28mol), then 18g of deionized water was added, and the solution was stirred for 1 hour. The solution changed from red to nearly colorless in the lower layer, and the organic solvent layer was yellow. Static to separate layers, spin off dichloroethane, suction filtration, wash with water, and vacuum dry to obtain 4.4 g of pale yellow solid 3,3',5,5'-tetra-tert-butyl-4,4'-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com