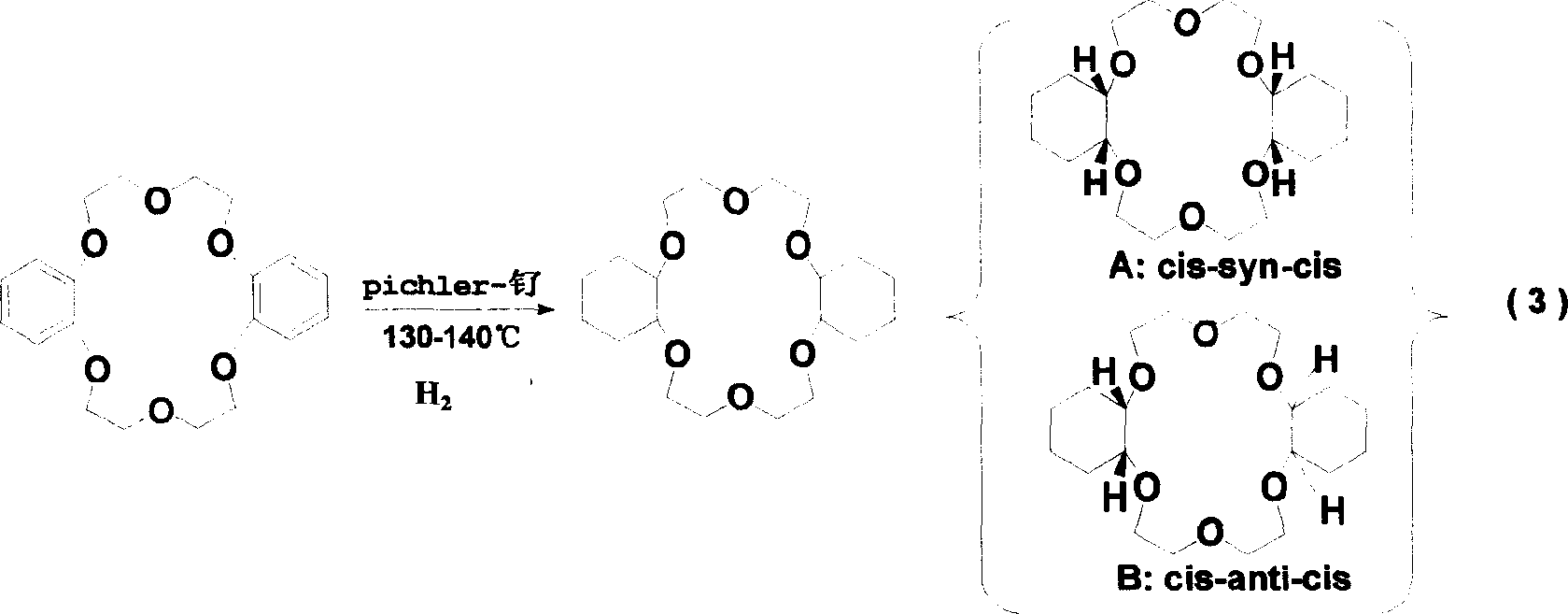
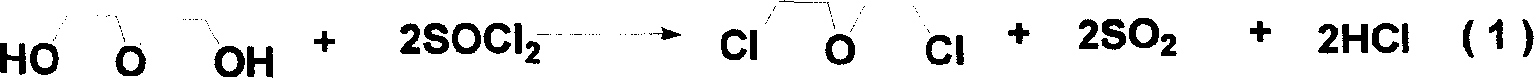Improved systhesizing process for dicyclohexyl-18-crown-6
A technology of dicyclohexyl and synthesis process, applied in the field of preparation of crown ether compounds, can solve the problems of low synthesis yield, low content of active ingredient DCH18C6 cis-stereoisomer, etc., and achieve the effect of shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] 1) Preparation of intermediate bis(2-chloroethyl)ether: add thionyl chloride to diethylene glycol, the molar ratio of the two is (2~3):1, and rapidly heat up to 110~120°C for reaction, The acid gas produced by the reaction is introduced into the lye for absorption, and the reaction is completed when no acid gas escapes, and the bis(2-chloroethyl)ether product is obtained by distillation under reduced pressure;
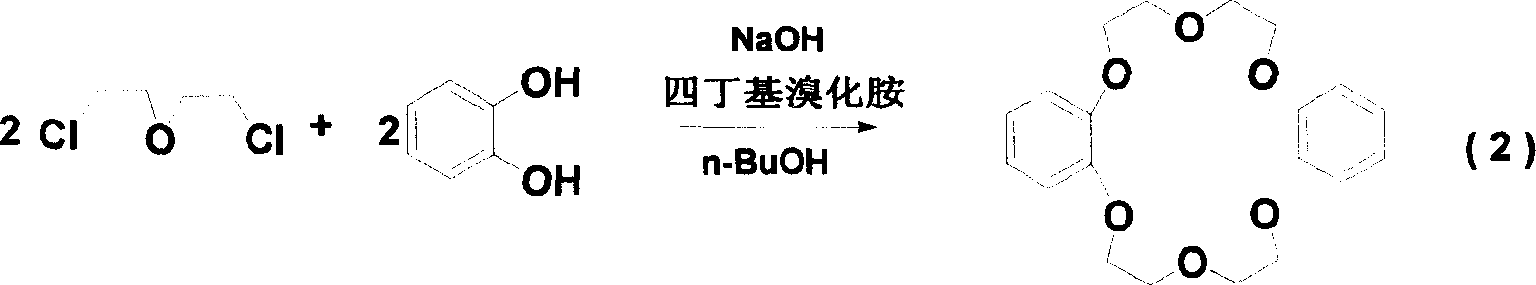
[0022] 2) Preparation of the intermediate dibenzo-18-crown 6: catechol, n-butanol, and tetrabutylammonium bromide are added at one time, and the molar ratio of the three is 1: (8-11): (0.02-0.04); Sodium oxide and bis(2-chloroethyl)ether are added in twice, and the amount of the first addition can be 20-80% (mass). Wherein the molar ratio of sodium hydroxide and bis(2-chloroethyl) ether to catechol is (4.1~4.5):(1~1.2):1, and the reflux reaction time is more than 10 hours, preferably 11~15 hours . After the system is acidified to acidity, n-butanol is distille...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com