Spinel type composite metal oxide electrode material and preparing process thereof
A composite metal and spinel-type technology, which is applied in electrode manufacturing, chemical instruments and methods, iron compounds, etc., can solve problems such as high cost, difficult industrialization, and complicated process, and achieve low energy consumption, convenient operation, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] CoSO 4 ·7H 2 O, FeSO 4 ·7H 2 O, Fe 2 (SO 4 ) 3 Moore Co 2+ / Fe 2+ / Fe 3+ = 3 / 5 / 2 ratio mixed, dissolved by N 2 In deionized water, make a salt solution with a total concentration of metal ions of 0.8mol / L, in N 2 Under protection, the NaOH solution with a concentration of 1.5 mol / L was slowly added dropwise to the salt solution, and when the pH value was 8.0, the dropwise addition of lye was stopped. The reactant was crystallized in a water bath at 50° C. for 3 hours, and the reaction was terminated. by N 2 Wash the product with degassed ice water, and then wash with ethanol at 0-5°C to remove unreacted metal ions, and dry the sample in air.
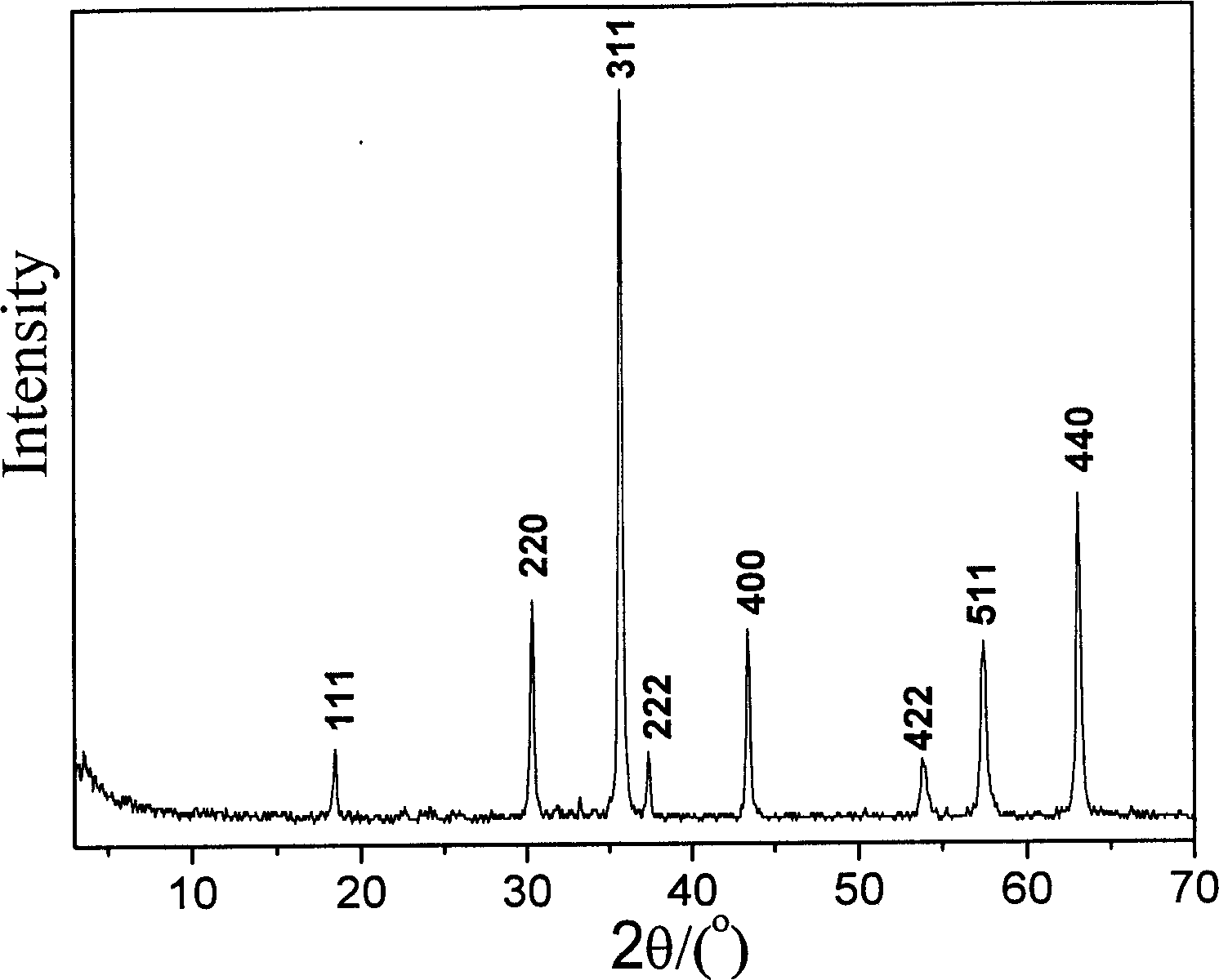
[0017] The dried Co-Fe 2+ -Fe 3+ -LDHs were placed in a muffle furnace, heated to 800°C at a rate of 10°C / min, kept for 1 hour, and cooled to room temperature with the furnace. The content of metal ions in the product was measured by Japan Shimadzu ICPS-7500 inductively coupled plasma emission spectrometer, and its ...
Embodiment 2
[0019] NiSO 4 ·6H 2 O, FeSO 4 ·7H 2 O, Fe 2 (SO 4 ) 3 Moore than Ni 2+ / Fe 2+ / Fe 3+ = 3 / 5 / 2 ratio mixed, dissolved by N 2 In deionized water, make a solution with a total concentration of metal ions of 1.0mol / L, in N 2Under protection, the NaOH solution with a concentration of 1.0 mol / L was slowly added dropwise to the salt solution, and when the pH value reached 6.5, the dropwise addition of lye was stopped. The reactant was crystallized in a water bath at 30° C. for 5 hours to complete the reaction. by N 2 Wash the product with degassed ice water, then wash with ethanol at 0-5°C to remove unreacted metal ions, and dry the sample in air.
[0020] The dried Ni-Fe 2+ -Fe 3+ - LDHs were placed in a muffle furnace, heated to 650°C at a rate of 5°C / min, kept for 3 hours, then cooled to room temperature with the furnace, ICP and XRD tests showed that the product was spinel NiFe with a single crystal phase 2 o 4 .
Embodiment 3
[0022] NiSO 4 ·6H 2 O, ZnSO 4 ·7H 2 O, FeSO 4 ·7H 2 O, Fe 2 (SO 4 ) 3 Moore than Ni 2+ / Zn 2+ / Fe 2+ / Fe 3+ = 1 / 2 / 5 / 2 ratio mixed, dissolved by N 2 In deionized water, make a solution with a total concentration of metal ions of 0.8mol / L, in N 2 Under protection, the NaOH solution with a concentration of 1.5 mol / L was slowly added dropwise to the salt solution, and when the pH value reached 7, the dropwise addition of lye was stopped. The reactant was crystallized in a water bath at 40° C. for 4 hours, and the reaction was terminated. by N 2 Wash the product with degassed ice water, then wash with ethanol at 0-5°C to remove unreacted metal ions, and dry the sample in air.
[0023] The dried Ni-Zn-Fe 2+ -Fe 3+ -LDHs were placed in a muffle furnace, heated to 700°C at a rate of 10°C / min, kept for 2 hours, then cooled to room temperature with the furnace, ICP and XRD tests showed that the product was spinel Ni with a single crystal phase 1 / 3 Zn 2 / 3 Fe 2 o 4 . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com