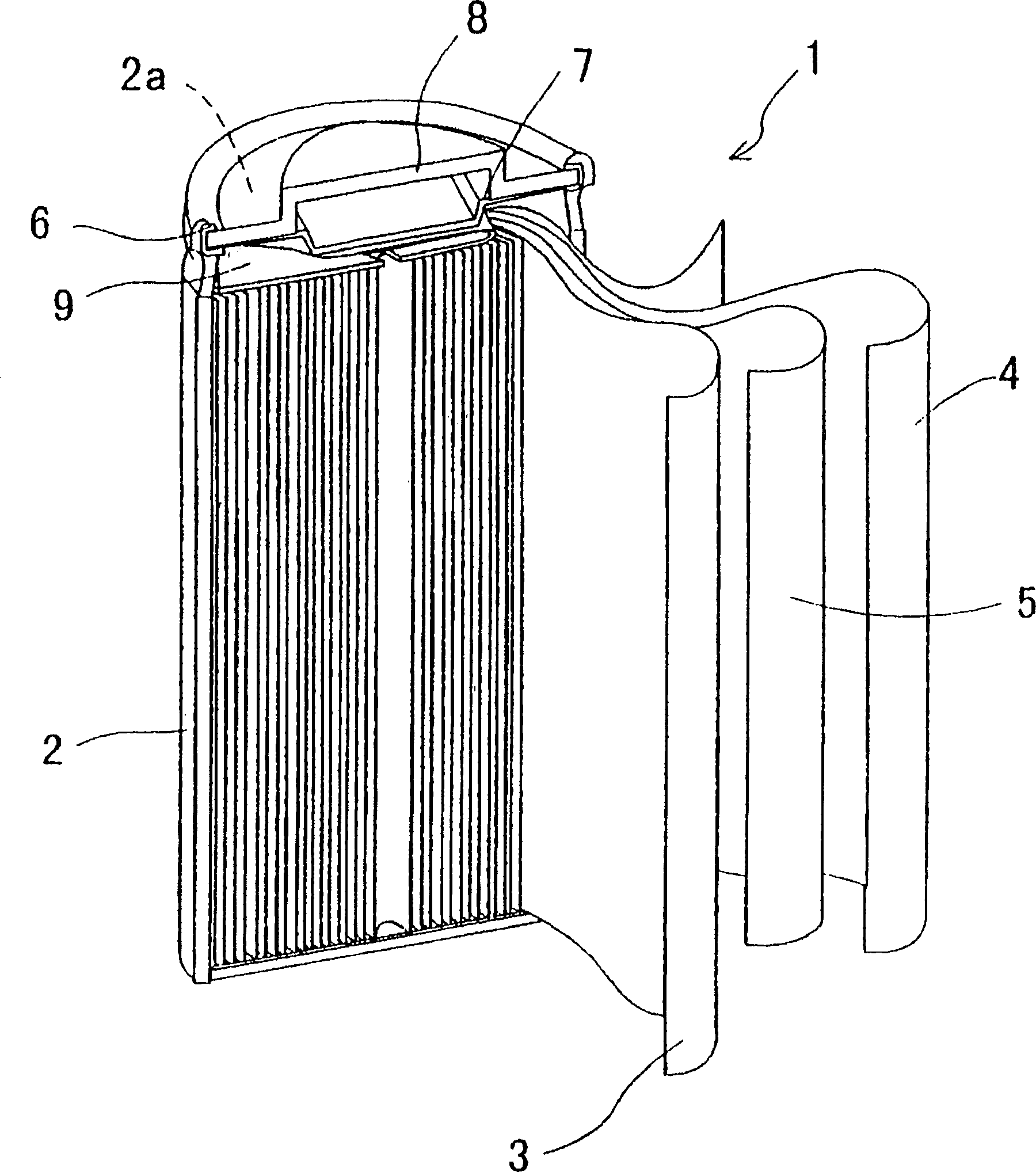
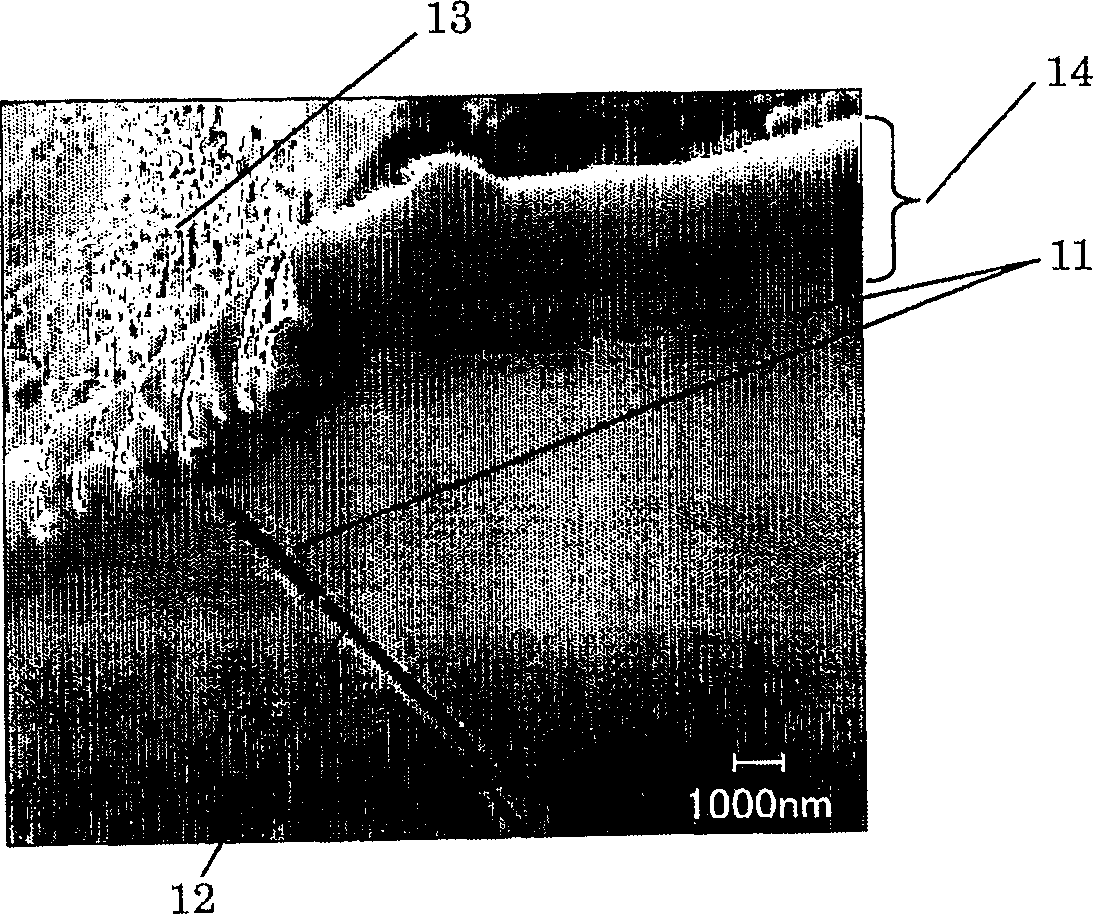
Closed nickel-hydrogen storage battery and its production method
A nickel-metal hydride storage battery and a manufacturing method technology, applied to nickel storage batteries, alkaline storage batteries, battery electrodes, etc., can solve problems such as ineffective effects, and achieve the effects of improving performance, expanding application range, and good cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0079] Synthesis of Nickel Hydroxide Particles
[0080] Nickel sulfate, zinc sulfate, and cobalt sulfate are dissolved in a mass ratio of metal hydroxides to be described later to form an aqueous solution, and ammonium sulfate and sodium hydroxide aqueous solution are added to the aqueous solution to form an ammonium complex. When the reaction system was stirred rapidly, further drip sodium hydroxide solution, the temperature of the reaction bath was controlled at 45 ± 2°C, the pH value was controlled at 12 ± 0.2, and the mass ratio of hydroxide was nickel: zinc: cobalt = 93:5:2 to synthesize spherical high-density nickel hydroxide particles constituting the core base material.
[0081] Formation of a surface layer on the surface of nickel hydroxide particles
[0082] Put the above-mentioned high-density nickel hydroxide particles into an alkaline aqueous solution whose pH is controlled by sodium hydroxide to 12±0.2. While stirring this solution, an aqueous solution containi...
Embodiment 6~10
[0118] Table 4 shows the necessary treatment time for alloys obtained in the same manner as alloy 1 of the present invention except that the treatment time was controlled so that the mass saturation magnetization (unit emu / g) was 1.0, 2.5, 4.0, 5.0, 8.0, 9.0, and 10.0. Among them, the alloys whose mass saturation magnetization (unit emu / g) is 1.0, 2.5, 4.0, 5.0, 8.0, and 9.0 are set as the alloy 6 of the present invention, the alloy 7 of the present invention, the alloy 8 of the present invention, the alloy 1 of the present invention, and the alloy of the present invention. Invention Alloy 9 and Invention Alloy 10, the formed batteries are set as Invention Battery 6, Invention Battery 7, Invention Battery 8, Invention Battery 1, Invention Battery 9, and Invention Battery 10. In addition, an alloy with a mass saturation magnetization of 0.5 and an alloy with a mass saturation magnetization of 10.0 that were not treated were set as Comparative Alloy 4 and Comparative Alloy 5, and...
Embodiment 11~13
[0126] The alloy powder obtained in the same manner as in Example 1 was Alloy 11 of the present invention except that 5% of yttrium was added to the Mm (cerium-lanthanum alloy) composition of the hydrogen storage alloy, and it was the same as in Example 1 except that 5% of ytterbium was added. The alloy powder thus obtained is Alloy 12 of the present invention, and the alloy obtained in the same manner as in Example 1 is Alloy 13 of the present invention except that 5% of erbium is added. Battery 12, battery 13 of the present invention. The results are shown in Table 5.
[0127] differentiate
processing time
(h)
Negative board discharge capacitor
Quantity (mAh)
10Ita discharge
Discharge capacity (%)
Cycle times
number (cycle)
Example 1
0
290
80
800
Example 11
5
286
85
1030
Example 12
10
286
81
900
Example 13
20
285
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com