End capped thiofuran small-molecule and polymer material, treating method and use thereof
A technology for thiophene and compounds, which is applied in the field of thiophene small molecules and polymer materials and their processing, and can solve problems such as complex preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
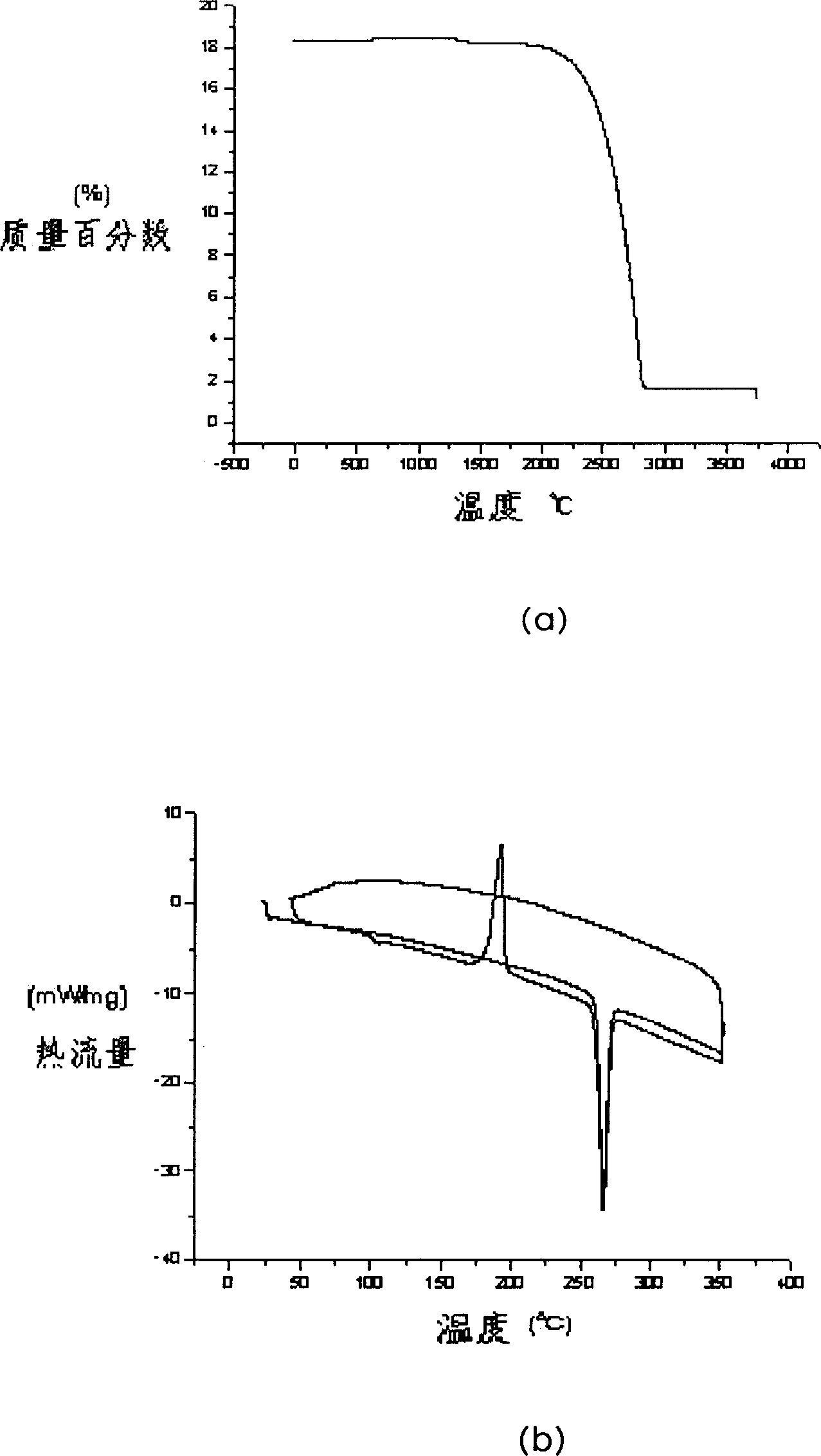
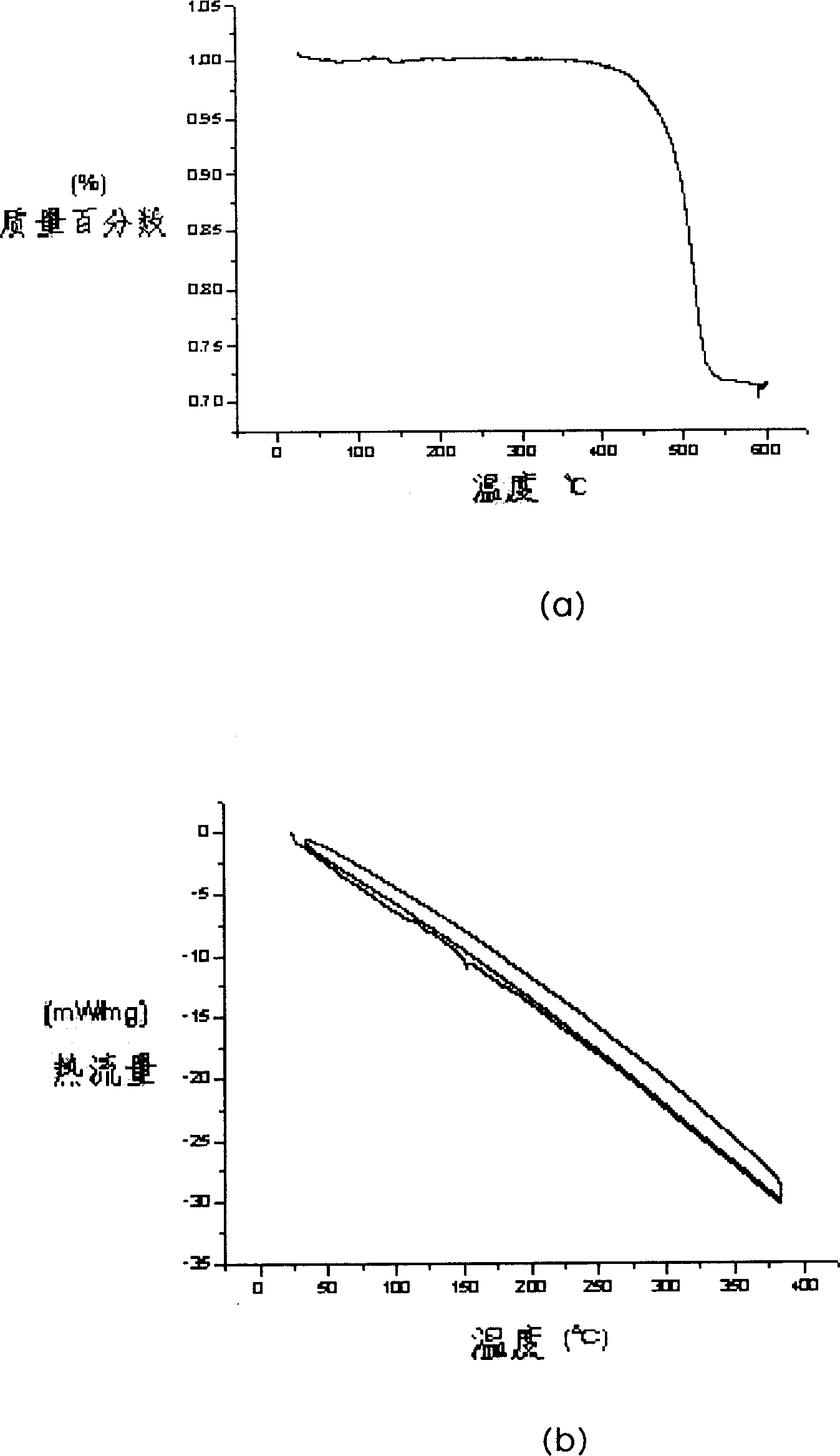
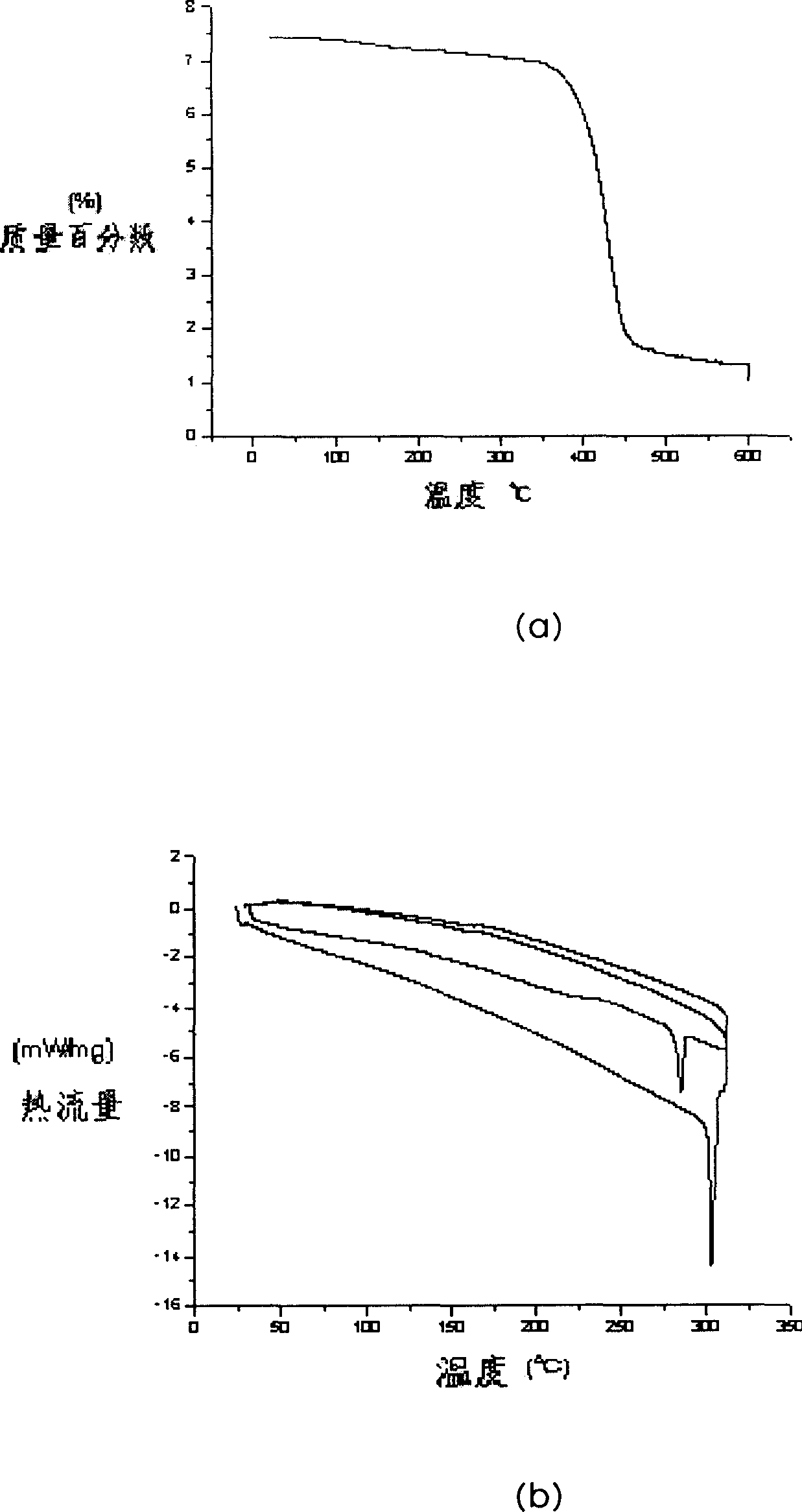
Image
Examples
Embodiment 1
[0072] Example 1, 9-phenyl-fluoren-9-ol as a capping agent for the preparation of capping materials for thiophene:
[0073] 9-Phenyl-fluoren-9-ol
[0074] 9-phenyl-9H-fluoren-9-ol
[0075] Take bromo-benzene (2.1mmol) and magnesium Mg (0.502g, 2.1mmol) to react to generate Grignard reagent, and react with fluorenone (2.1mmol) dissolved in 16mL tetrahydrofuran at 60°C for 24 hours to form a large amount of white precipitate, and finally add Saturated color NHCl 4 Convert Grignard salts to alcohols. After the reaction was completed, ether was extracted, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain a slightly pale yellow solid tertiary alcohol (yield 90%).
[0076] GC-MS (EI-m / z): 258.1 (M + ).
[0077] 1 H NMR (400MHz, CDCl 3 , ppm): δ7.691-7.672 (d, J=7.6Hz, 2H), 7.406-7.325 (m, 6H), 7.292-7.236 (m, 5H), 2.508 (s, 1H).
[0078] 13 C NMR (CDCl 3 , ppm): δ150.658, 143.391, 139.82...
Embodiment 2
[0084] Example 2, 9-phenyl-fluoren-9-ol as a capping agent to treat 2,2'-dithiophene capping material preparation:
[0085] 5,5'-bis(9-phenyl-fluoren-9-yl)-2,2'-dithiophene
[0086] 5,5′-bis(9-phenyl-fluoren-9-yl)-2,2′-Bithiophene
[0087] Dissolve 9-phenyl-fluorene-9-ol and 2,2'-dithiophene in dichloromethane at a ratio of 2:1, add boron trifluoride-ether complex dropwise at room temperature for 30 minutes , adding ethanol and water to quench the reaction, dichloromethane extraction, drying and rotary evaporation, petroleum ether: dichloromethane silica gel column purification, recrystallization with tetrahydrofuran and petroleum ether to obtain white powder solid 5,5'bis(9-phenyl- Fluoren-9-yl)-2,2'-dithiophene (92.1% yield).
[0088] MALDI-TOF-MS (m / z): 646.2 (M + ).
[0089] 1 H NMR (400MHz, CDCl 3 , ppm): δ7.759-7.74 (d, J=7.6Hz, 4H), 7.491-7.472 (d, J=7.6Hz, 4H), 7.392-7.352 (td, J=7.2Hz, J=1.2Hz, 4H), 7.303-7.257(td, J=7.6Hz, J=1.2Hz, 4H), 7.228-7.188(m, 10H), 6....
Embodiment 3
[0091] Example 3, 9-phenyl-fluoren-9-ol used as a capping agent to treat 3,3'-dithiophene capping material preparation:
[0092] 2,5,2',5'-tetrakis(9-phenyl-fluoren-9-yl)-3,3'-dithiophene
[0093] 2,5,2',5'-tetrakis(9-phenyl-fluoren-9-yl)-3,3'-bithiophene
[0094] Dissolve 9-phenyl-fluorene-9-ol and 3,3'-dithiophene in dichloromethane at a ratio of 4:1, and add boron trifluoride-diethyl ether complex dropwise at room temperature for 30 minutes , adding ethanol and water to quench the reaction, dichloromethane extraction, drying and rotary evaporation, petroleum ether: dichloromethane silica gel column purification, recrystallization with tetrahydrofuran and petroleum ether to obtain white powder solid 2,5,2′,5′-tetra (9-Phenyl-fluoren-9-yl)-3,3'-dithiophene (81.2% yield).
[0095] MALDI-TOF-MS (m / z): 1126.6 (M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com