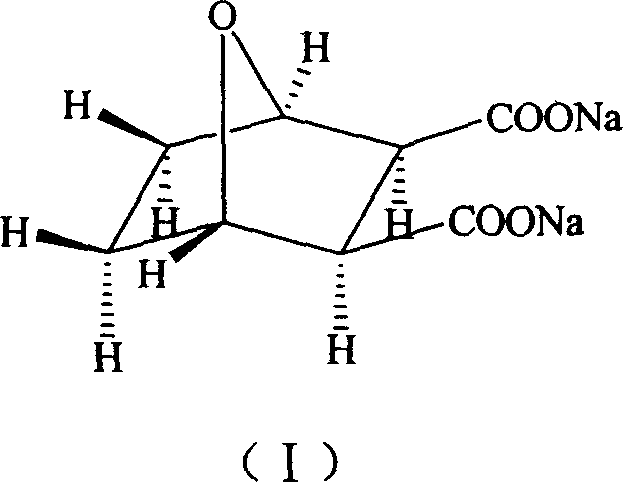
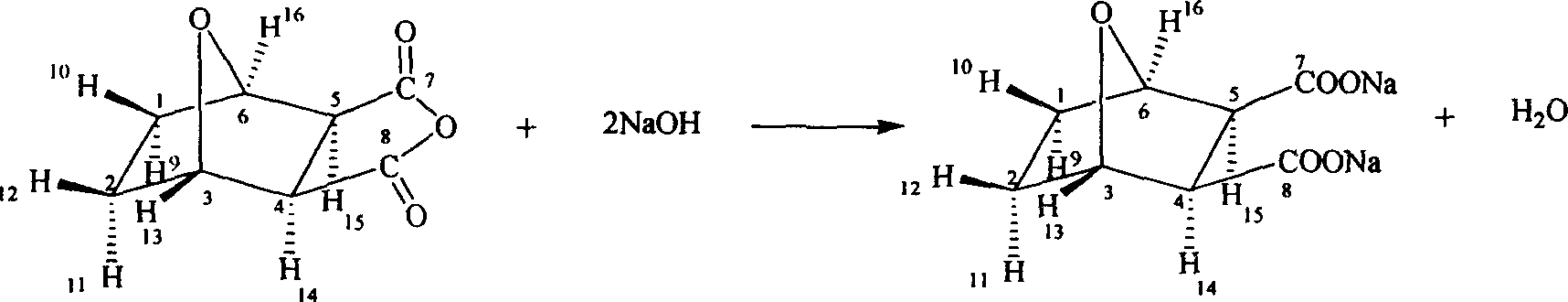
Demethyl sodium cartharidate medicinal compound and its preparation method and application
A technology of sodium norcantharidate and a pharmaceutical compound is applied in the field of sodium norcantharidate pharmaceutical compound for medicine, and achieves the effects of convenient synthesis, huge application prospect and good water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: norcantharidate sodium synthesis
[0019] At room temperature, norcantharidin (100g, 0.60mol) and sodium hydroxide (47.6g, 1.20mol) are added in the reaction flask, water (300ml) is added, and the stirring reaction is no longer changed until the pH is added, and activated carbon for needles ( 1.5 g), stirred for 30 minutes, filtered, the filtrate was evaporated to dryness at 120°C, and dried in vacuum at 80°C to obtain 135g of the product, with a yield of 98.5%.
[0020] Appearance: off-white crystalline powder
[0021] Content: 100%
[0022] HPLC method: chromatographic column is YWG-C 18 (5μm, 4.6mm×250mm), the mobile phase is 0.025mol L -1 Potassium dihydrogen phosphate solution-methanol (75:25) (pH3.0), the detection wavelength is 210nm.
[0023] pH: 8.5 (10% aqueous solution)
[0024] IR(KBr)υ max : 2995, 2965, 2948, 2910, 1609, 1609, 1589, 1434, 1405.
[0025] 1 H-NMR (D 2 O)δ: 4.60(s, H-13, H-16), 2.76(s, H-14, H-15), 1.56(m, H-10, H-12), 1...
Embodiment 2
[0029] Embodiment 2: norcantharidate sodium synthesis
[0030] At 60°C, add norcantharidin (100g, 0.60mol) and sodium hydroxide (47.6g, 1.20mol) into the reaction flask, add water (300ml), stir the reaction until the pH does not change, add activated carbon for needles (1.5g), stirred for 30 minutes, filtered, the filtrate was evaporated to dryness at 140°C, and vacuum-dried at 80°C to obtain 136g of the product, with a yield of 99.2%.
[0031] Appearance: off-white crystalline powder
[0032] Content: 100%
[0033] HPLC method: chromatographic column is YWG-C 18 (5μm, 4.6mm×250mm), the mobile phase is 0.025mol L -1 Potassium dihydrogen phosphate solution-methanol (75:25) (pH3.0), the detection wavelength is 210nm pH: 8.7 (10% aqueous solution) IR (KBr) υ max : 2998, 2970, 2945, 2911, 1610, 1585, 1430, 1408.
[0034] 1 H-NMR (D 2 O)δ: 4.60(s, H-13, H-16), 2.76(s, H-14, H-15), 1.56(m, H-10, H-12), 1.42(m, H-9 , H-11).
[0035] 13 C-NMR (D 2 O)δ: 180.1 (C-7, C-8), 79....
Embodiment 3
[0037] Embodiment 3: preparation of sodium norcantharidate injection
[0038] Prescription: Sodium Norcantharidate 13.7g
[0039] Add water for injection to 2000ml
[0040] Made 1000 pieces
[0041] Preparation:
[0042] (1) The ampoule is washed clean, dried and sterilized;
[0043] (2) Take by weighing norcantharidin sodium 13.7g, add prescription amount 90% water for injection, stir and dissolve;
[0044] (3) The medicinal liquid is adsorbed with 0.05% (w / v) medicinal charcoal for 30 minutes, and decarbonized and filtered;
[0045] (4) Add water for injection to the prescribed amount, and filter with a 0.22 μm microporous membrane;
[0046] (5) Inspection of intermediate products;
[0047] (6) Filling, 2ml each, melt-sealed;
[0048] (7) Sterilize with circulating steam at 100°C for 30 minutes;
[0049] (8) Full inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com